
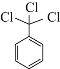
����Ŀ��������������һ�ֹ���������Ƽ����ܱ�ҶƬ�Ͽ�����գ�����ֲ�����ڴ����ٶȽ���������ѿǰ���ݼ�����Ҫ���ڴ��ݵȡ���ҵ��ͨ������A���кϳɣ���ϳ�·�����£�
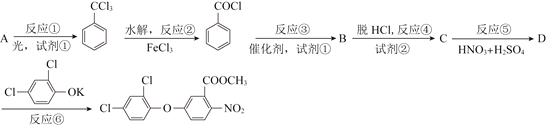
��֪��![]()
![]() RCH=O+H2O(R��������)
RCH=O+H2O(R��������)
(1)�Լ���Ϊ��_____________���Լ���Ϊ___________��
(2)д��A�Ľṹ��ʽ_____________��
(3)д�����з�Ӧ�Ļ�ѧ����ʽ����Ӧ��____________����Ӧ��____________��
(4)���ڱ����Ͳ������ŵ��Ӱ�죬�����������Ļ����ڱ�����ȡ����λ����ԭ�л��ž������磺���ӷ�������![]() ��ʹ������_________
��ʹ������_________![]() ѡ�����գ���ͬ
ѡ�����գ���ͬ![]() ��Hԭ�����ױ�ȡ����������Ŀ��Ϣ��֪��
��Hԭ�����ױ�ȡ����������Ŀ��Ϣ��֪��![]() ��ʹ������________��Hԭ�����ױ�ȡ����
��ʹ������________��Hԭ�����ױ�ȡ����
![]() ��λ
��λ ![]() ��λ
��λ ![]() ��λ
��λ
(5)��Ӧ�����ڱ���![]() ��Һ�н��У�����NaOH��Һ�н��У�����һ��ˮ�⣬��д��
��Һ�н��У�����NaOH��Һ�н��У�����һ��ˮ�⣬��д��![]() ������NaOH��Һ����ȫˮ��Ļ�ѧ��Ӧ����ʽ_________________
������NaOH��Һ����ȫˮ��Ļ�ѧ��Ӧ����ʽ_________________
���𰸡����� �״� ![]()
![]()
![]()
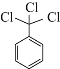
![]()
![]() +HNO3
+HNO3![]()
![]() +H2O
+H2O ![]() b
b ![]()
![]()
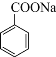
![]()
��������
����A�ڹ�������������![]() ����֪AΪ
����֪AΪ![]() ���ٽ��D��
���ٽ��D��![]() ����
����![]() ��֪DΪ
��֪DΪ![]() ��C�����ᣬ���������µĵ�D����֪CΪ��
��C�����ᣬ���������µĵ�D����֪CΪ��![]() ������C����BΪ
������C����BΪ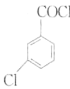 ���Լ�
���Լ�![]() Ϊ�״���
Ϊ�״���
![]() �ɷ�����֪�Լ�
�ɷ�����֪�Լ�![]() Ϊ�������Լ�
Ϊ�������Լ�![]() Ϊ�״���
Ϊ�״���
�ʴ�Ϊ��Ϊ������Ϊ�״���
![]() �ɷ�����֪A�Ľṹ��ʽ
�ɷ�����֪A�Ľṹ��ʽ![]() ��
��
�ʴ�Ϊ��![]() ��
��
![]() �ɷ�����֪����ʽΪ��
�ɷ�����֪����ʽΪ��![]()
![]()
![]()
![]()
![]() ��
��![]() +HNO3
+HNO3![]()
![]() +H2O��
+H2O��
�ʴ�Ϊ��![]()
![]()
![]()

![]() ��
��![]() +HNO3
+HNO3![]()
![]() +H2O��
+H2O��
![]() ���ڱ����Ͳ������ŵ��Ӱ�죬�����������Ļ����ڱ�����ȡ����λ����ԭ�л��ž������磺���ӷ�������
���ڱ����Ͳ������ŵ��Ӱ�죬�����������Ļ����ڱ�����ȡ����λ����ԭ�л��ž������磺���ӷ�������![]() ��ʹ�����ϵ��ڶ�λ��û��ã��ɷ�Ӧ
��ʹ�����ϵ��ڶ�λ��û��ã��ɷ�Ӧ![]() ��֪��
��֪��![]() ��ʹ�����ϼ�λ��Hԭ�����ױ�ȡ����
��ʹ�����ϼ�λ��Hԭ�����ױ�ȡ����
�ʴ�Ϊ��ac��b��
![]() ��Ӧ
��Ӧ![]() ����NaOH��Һ�н��л�һ�����ɱ������ƣ�����ʽΪ��
����NaOH��Һ�н��л�һ�����ɱ������ƣ�����ʽΪ��![]() +4NaOH��
+4NaOH��![]()
![]() ��
��
�ʴ�Ϊ��![]() +4NaOH��
+4NaOH��![]()
![]() ��
��


 ����ѧ���ʱѧ����ϵ�д�
����ѧ���ʱѧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��ڼ۵����Ų�ʽΪ3s23p4������������ȷ���ǣ� ��
A.����Ԫ�ط���ΪO
B.���ĺ�������Ų�ʽΪ1s22s22p63s23p4
C.������H2����Һ̬������
D.������Ų�ͼΪ![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����( )
A.��Ӧ2Mg��CO2![]() 2MgO��C ��H��0���ر�Ƕȿ������Է�����
2MgO��C ��H��0���ر�Ƕȿ������Է�����
B.���ܱ������������淴Ӧ��2NO(g)��2CO(g)![]() N2(g)��2CO2(g) ��H����113.0 kJ/mol���ﵽƽ������¶Ȳ��䣬��С������������´ﵽƽ�����H��С
N2(g)��2CO2(g) ��H����113.0 kJ/mol���ﵽƽ������¶Ȳ��䣬��С������������´ﵽƽ�����H��С
C.��֪��Ksp(AgCl)��1.8��10��10��Ksp(Ag2CrO4)��2.0��10��12���������Ũ��Ϊ1.0��10��4mol/L��AgNO3��Һ���뵽Ũ�Ⱦ�Ϊ1.0��10��4mol/L��KCl��K2CrO4�Ļ����Һ�в������ֲ�ͬ��������Ag2CrO4�����Ȳ���
D.����HClO��Ka��3.0��10��8��H2CO3��Ka1��4.3��10��7�� Ka2��5.6��10��11�����Ʋ���ͬ״���£���Ũ�ȵ�NaClO��Na2CO3��Һ�У�pHǰ��С�ں���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й����л��������˵��������ǣ� ��
A.����������ڹ��������·�Ӧ�IJ�����5��
B.2���һ���1��3������ϩ���ӵļ���ʽ��![]()
C.����ʽΪC5H12O2�Ķ�Ԫ��������̼ԭ����Ϊ3�Ľṹ��2��
D.Ϊ�����ȱ����е���Ԫ�أ��ɽ��ȱ�����NaOH��Һ���ȼ����Ӻ���ȴ���μ�AgNO3��Һ���۲��Ƿ��а�ɫ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������־����־�����������й��ڰ�ͭ�ļ��أ���������ͭ(ͭ���Ͻ�)�������⣬����Ҫ������ң������������������Ʒ���ش��������⣺
(1)��Ԫ�ػ�̬ԭ�ӵļ۵��ӹ����ʾʽ___��3d�ܼ��ϵ�δ�ɶԵĵ�����Ϊ___��
(2)���������ڰ�ˮ�γ�[Ni(NH3)6]SO4��ɫ��Һ��
��[Ni(NH3)6]SO4�������ӵ����幹����___��
����[Ni(NH3)6]2+��Ni2+��NH3֮���γɵĻ�ѧ����Ϊ___���ṩ�µ��ӶԵijɼ�ԭ����___��
�۰��ķе�_______(������������������)�(PH3)��ԭ����______��
(3)����ͭ����������__���γɵľ��壺Ԫ��ͬ�����ĵڶ������ֱܷ�Ϊ��ICu=1959kJ/mol��INi=1753kJ/mol��ICu>INi��ԭ����___��
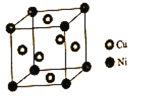
(4)ij����ͭ�Ͻ�����������ṹ��ͼ��ʾ��
�پ�����ͭԭ������ԭ�ӵ�������Ϊ___��
�����Ͻ���ܶ�Ϊdg/cm3����������a=____nm��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼʾ���Ӧ�������������

ͼ1 ͼ2 ͼ3 ͼ4
A. ͼ1��ʾ0.1molMgCl2��6H2O�ڿ����г�ּ���ʱ����������ʱ��ı仯
B. ͼ2��ʾ��0.1000 mol��L��lNaOH��Һ�ζ�25.00 mLCH3COOH�ĵζ����ߣ���c(CH3COOH)��0.0800 mol��L��1
C. ͼ3��ʾ���º��������£�2NO2(g)![]() N2O4(g)�У������ʵ�Ũ��������������֮��Ĺ�ϵ�����н���A��Ӧ��״̬Ϊ��ѧƽ��״̬
N2O4(g)�У������ʵ�Ũ��������������֮��Ĺ�ϵ�����н���A��Ӧ��״̬Ϊ��ѧƽ��״̬
D. ͼ4��ʾ�����£�ϡ��HA��HB�������ϡ��Һʱ����ҺpH���ˮ���ı仯�������£�NaA��Һ��pHС��ͬŨ�ȵ�NaB��Һ��pH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������Ԫ�أ�����A��B��C��D��EΪ��������Ҫ��Ԫ�أ�FΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⡣
AԪ���γɵ���������࣬���γɵ�һ�ֹ��嵥�ʹ�ҵ�ϳ������и�� |
BԪ��ԭ�ӵĺ���p��������s��������1 |
CԪ�ػ�̬ԭ��p���������δ�ɶԵ��� |
Dԭ�ӵĵ�һ�����ĵ����ֱܷ��ǣ� ��1=738kJ��mol-1����2=1451kJ��mol-1����3=7733kJ��mol-1����4=10540kJ��mol-1�� |
Eԭ�Ӻ�������p���ȫ������� |
F�����ڱ��ĵ�8���� |
��1��ijͬѧ����������Ϣ���ƶ�A��̬ԭ�ӵĺ��������Ų�Ϊ��![]() ����ͬѧ�����ĵ����Ų�ͼΥ����___________��
����ͬѧ�����ĵ����Ų�ͼΥ����___________��
��2��BԪ�صĵ縺��_____������ڡ�����С�ڡ����ڡ���CԪ�صĵ縺�ԡ�
��3��C��D�γɵĻ����������еĻ�ѧ������Ϊ_____________��
��4��E��̬ԭ����������ߵĵ��ӣ���������ڿռ���__________������
��5�����й���Fԭ�ӵļ۲�����Ų�ͼ��ȷ����___________��
a.![]() b.
b.![]()
c.![]() d.
d.![]()
��6����̬F3+���Ӻ�������Ų�ʽΪ_____________����������F��B������������ˮ�����ϡ��Һ��ȫ��Ӧ������BC���壬�÷�Ӧ�����ӷ���ʽΪ____________��
��7��Ԫ��ͭ�����ĵڶ������ֱܷ�Ϊ��ICu=1959kJ��mol-1,INi=1753kJ��mol-1,ICu��INiԭ����__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ѧ��ȤС��Ϊ�˵��鵱��ijһ������ˮ����Ⱦ�������ע�������3����ҪˮԴ����ڴ��ɼ�ˮ�����������˷���������������ʵ����Ϣ������һ��ˮԴ����A��B�������ʣ�һ������C��D�������ʣ�һ������E���ʣ�A��B��C��D��EΪ���ֳ�������������±��е������γɣ�
������ | K+ Na+ Cu2+ Al3+ |
������ | SO42�� HCO3- NO3- OH�� |
Ϊ�˼�������������ֱ��������ʵ�飬�����ǣ�
�ٽ���������ˮ��DΪ��ɫ��Һ��������Ϊ��ɫ��Һ��
�ڽ�E��Һ���뵽C��Һ�г��ְ�ɫ�����������μӣ������ܽ⣻
�۽�����ɫ��Ӧ��ֻ��B��CΪ��ɫ������ɫ�ܲ�������
���ڸ���Һ�м������ᱵ��Һ���ټӹ���ϡ���ᣬA�зų���ɫ���壬C��D�ж��ܲ�����ɫ������
�ݽ�B��D����Һ��ϣ�δ���������������ɡ�
��������ʵ����գ�
��1��д��C��D���Ļ�ѧʽ��C_______��D______��
��2������1 mol A����Һ�뺬l mol E����Һ��Ӧ�����ɣ����õ�һ�ֻ������д��A��E��Ӧ�����ӷ���ʽ��_______________��
��3����A��Һ�м�����������ʯ��ˮ�������ӷ���ʽΪ_____________________��
��4��C��������ˮ���������ӷ���ʽ���ʵ�����˵���侻ˮԭ��______________________��
��5����������0.5 mol��C��Һ����μ���Ba(OH)2��Һ�����ɳ����������Ϊ__________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ش����й���NO��NO2�����⣺
(1)������������װ�д�ת�����ɼ���β���Ի�������Ⱦ������β���е��к�����CO��NO��Ӧ��ת��Ϊ�������ŷţ�д����ط�Ӧ�Ļ�ѧ����ʽ��___________________
(2)��ҵ���������ð�ˮ����SO2��NO2��ԭ����ͼ��ʾ��
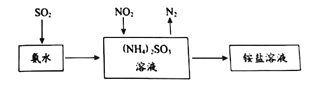
NO2�����պ����ɵ������____________(�ѧʽ)��Ϊ֤����Һ��NH4+�Ĵ��ڣ��������������Һ�м���___________��Һ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com