
��5�֣�ijʵ��С���H2O2�ķֽ���������̽����
��1���±��Ǹ�ʵ��С���о�Ӱ��H2O2�ֽ����ʵ�����ʱ��¼��һ�����ݣ�
��10ml H2O2��ȡ150ml����״���£�O2�����ʱ�䣨s��
| ��Ӧ���� ʱ�䣨s�� ��Ӧ���� ʱ�䣨s�� Ũ�� |
30%H2O2 |
15%H2O2 |
10%H2O2 |
5%H2O2 |
| ������������ | ��������Ӧ | ��������Ӧ | ��������Ӧ | ��������Ӧ |
| ���������� | 360 | 480 | t | 720 |
| MnO2���������� | 10 | 25 | 60 | 120 |
�ٸ�ʵ��С������Ʒ���ʱ��������Ũ�ȡ� �� �����ض�H2O2�ֽ����ʵ�Ӱ�졣
![]() ���Ʋ�t�ķ�ΧΪ ��
���Ʋ�t�ķ�ΧΪ ��
��2����������ͬ���ۼ�״̬��ͬ��MnO2�ֱ����15 ml 5%��H2O2��Һ�У����ô����ǵ�ľ�����ԣ�������£�
| ������MnO2�� | ������� | �۲��� | ��Ӧ��������ʱ�� |
| ��ĩ״ | ��ϲ��� | ���ҷ�Ӧ�������ǵ�ľ����ȼ | 3.5min |
| ��״ | ��Ӧ���������Ǻ�����ľ��δ��ȼ | 30min |
��д������ʵ���з�����Ӧ�Ļ�ѧ����ʽ��
��ʵ���������������Ĵ�Ч���� �йء�
��1�����¶ȡ�������480<t<720 ��2H2O2��2H2O+O2���ڴ����Ŀ�����С
����:������������Է�Ӧ���ʵ�Ӱ��
��1�����ݱ��е�ʵ�����ݿ��жϣ������ʵ�鷽��ʱ������Ũ�ȡ��¶Ⱥʹ����ȶԷ�Ӧ���ʵ�Ӱ�졣��ΪŨ��Խ��Ӧ����Խ�죬����10����˫��ˮ�ֽ�������ͬ������ʱС�ڵ�ʱ��Ҫ����15����˫��ˮ����С��5����˫��ˮ����480<t<720��
��2��˫��ˮ���ȶ������ȷֽ�����������ˮ����ӦʽΪ2H2O2��2H2O+O2��������ʵ��������жϷ�ĩ״�Ĵ�����Ч���ã���˵�������Ĵ�Ч��������Ŀ�����С�й�ϵ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �Թ� | �¶� | ��������Ũ�� | ���� |
| a | ���£�25�棩 | 12% | �� |
| b | ˮԡ���ȣ�50�棩 | 4% | �� |
| c | ˮԡ���ȣ�50�棩 | 12% | �� |
| d | ���£�25�棩 | 4% | �� |
| ʵ���� | ��Ӧ�� | ���� |
| �� | 10mL2% H2O2��Һ | �� |
| �� | 10mL5% H2O2��Һ | �� |
| �� | 10mL5% H2O2��Һ | 1mL0.1mol?L-1FeCl3��Һ |
| �� | 10mL5% H2O2��Һ+����HCl��Һ | 1mL0.1mol?L-1FeCl3��Һ |
| �� | 10mL5% H2O2��Һ+����NaOH��Һ | 1mL0.1mol?L-1FeCl3��Һ |
 O2��+2H2O
O2��+2H2O O2��+2H2O
O2��+2H2O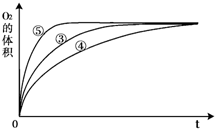
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ���� | ��Ӧ�� | ���� |
| �� | 10mL2% H2O2��Һ | �� |
| �� | 10mL5% H2O2��Һ | �� |
| �� | 10mL5% H2O2��Һ | 1mL0.1mol?L-1FeCl3��Һ |
| �� | 10mL5% H2O2��Һ+����HCl��Һ | 1mL0.1mol?L-1FeCl3��Һ |
| �� | 10mL5% H2O2��Һ+����NaOH��Һ | 1mL0.1mol?L-1FeCl3��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ĵ��ɶ������ѧУ��һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��5�֣�ijʵ��С���H2O2�ķֽ���������̽����
��1���±��Ǹ�ʵ��С���о�Ӱ��H2O2�ֽ����ʵ�����ʱ��¼��һ�����ݣ�
��10ml H2O2��ȡ150ml����״���£�O2�����ʱ�䣨s��
| ��Ӧ���� ʱ�䣨s�� ��Ӧ���� ʱ�䣨s�� Ũ�� | 30%H2O2 | 15%H2O2 | 10%H2O2 | 5%H2O2 |
| ������������ | ��������Ӧ | ��������Ӧ | ��������Ӧ | ��������Ӧ |
| ���������� | 360 | 480 | t | 720 |
| MnO2���������� | 10 | 25 | 60 | 120 |
 ���Ʋ�t�ķ�ΧΪ ��
���Ʋ�t�ķ�ΧΪ ��| ������MnO2�� | ������� | �۲��� | ��Ӧ��������ʱ�� |
| ��ĩ״ | ��ϲ��� | ���ҷ�Ӧ�������ǵ�ľ����ȼ | 3.5min |
| ��״ | ��Ӧ���������Ǻ�����ľ��δ��ȼ | 30min |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���Ĵ��ɶ������ѧУ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��5�֣�ijʵ��С���H2O2�ķֽ���������̽����
��1���±��Ǹ�ʵ��С���о�Ӱ��H2O2�ֽ����ʵ�����ʱ��¼��һ�����ݣ�
��10ml H2O2��ȡ150ml����״���£�O2�����ʱ�䣨s��
|
��Ӧ���� ʱ�䣨s�� ��Ӧ���� ʱ�䣨s�� Ũ�� |
30%H2O2 |
15%H2O2 |
10%H2O2 |
5%H2O2 |
|
������������ |
��������Ӧ |
��������Ӧ |
��������Ӧ |
��������Ӧ |
|
���������� |
360 |
480 |
t |
720 |
|
MnO2���������� |
10 |
25 |
60 |
120 |
�ٸ�ʵ��С������Ʒ���ʱ��������Ũ�ȡ� �� �����ض�H2O2�ֽ����ʵ�Ӱ�졣
 ���Ʋ�t�ķ�ΧΪ
��
���Ʋ�t�ķ�ΧΪ
��
��2����������ͬ���ۼ�״̬��ͬ��MnO2�ֱ����15 ml 5%��H2O2��Һ�У����ô����ǵ�ľ�����ԣ�������£�
|
������MnO2�� |
������� |
�۲��� |
��Ӧ��������ʱ�� |
|
��ĩ״ |
��ϲ��� |
���ҷ�Ӧ�������ǵ�ľ����ȼ |
3.5min |
|
��״ |
��Ӧ���������Ǻ�����ľ��δ��ȼ |
30min |
��д������ʵ���з�����Ӧ�Ļ�ѧ����ʽ��
��ʵ���������������Ĵ�Ч���� �йء�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com