
[��ѧ����ѡ��5���л��ﻯѧ����]��15�֣�
�߷��Ӳ���E�ͺ�����Ϣʹ�߷���ҩ��ĺϳ�������ͼ��ʾ��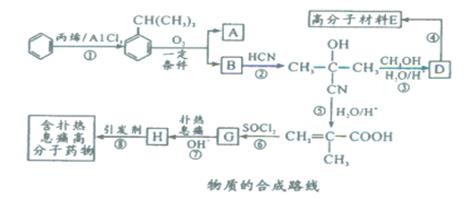
��֪��I��������Ϣʹ�߷���ҩ��ĽṹΪ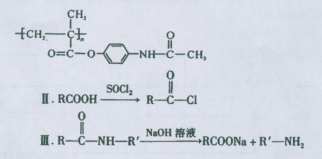
�Իش��������⣺
��1����Ӧ�ٵķ�Ӧ����Ϊ ��G�ķ���ʽΪ ��
��2����1mol CH�� CH3��2��ת��Ϊ1mol A��1mol B����A��FeCl3��Һ��������ɫ��д��A��ϡ��Һ�����Ũ��ˮ������Ӧ�Ļ�ѧ����ʽ�� ��
CH�� CH3��2��ת��Ϊ1mol A��1mol B����A��FeCl3��Һ��������ɫ��д��A��ϡ��Һ�����Ũ��ˮ������Ӧ�Ļ�ѧ����ʽ�� ��
��3����Ӧ��Ϊ�ӳɷ�Ӧ����B�Ľṹ��ʽΪ ������Ϣʹ�Ľṹ��ʽΪ ��
��4��D�����ܶ�����ͬ״̬�¼����ܶȵ�6��25����D�и�Ԫ�ص����������ֱ�Ϊ̼60%����8%����32%��D����������������Ϊ ��
��5��д��������Ϣʹ�߷���ҩ��������������Һ������Ӧ�Ļ�ѧ����ʽΪ ��
��6��D�ж���ͬ���칹�壬������D������ͬ���������ܷ���������Ӧ��ͬ���칹����
�֣�����˳���칹����
��������������ŷ�Ӧ��Ϊ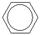 +CH2=CH-CH3 ��
+CH2=CH-CH3 ��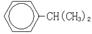 �����ڼӳɷ�Ӧ��������֪���֪G�Ľṹ��ʽΪCH2=C(CH3)-COCl�������ʽΪC4H5OCl��
�����ڼӳɷ�Ӧ��������֪���֪G�Ľṹ��ʽΪCH2=C(CH3)-COCl�������ʽΪC4H5OCl��
�ƾ����⡰ ��
��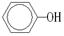 ��A��+
��A��+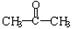 ��B��������A��ϡ��Һ�����Ũ��ˮ������Ӧ�Ļ�ѧ����ʽΪ��
��B��������A��ϡ��Һ�����Ũ��ˮ������Ӧ�Ļ�ѧ����ʽΪ��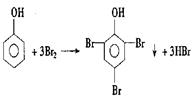 ��
��
�����ڷ�Ӧ��Ϊ�ӳɷ�Ӧ����B�Ľṹ��ʽΪ �����ݺ�����Ϣʹ�߷���ҩ��Ľṹ��ʽ ��
�����ݺ�����Ϣʹ�߷���ҩ��Ľṹ��ʽ ��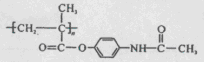 ����ȷ��H�Ľṹ��ʽΪ
����ȷ��H�Ľṹ��ʽΪ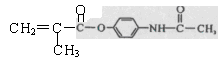 ���ٽ��G�Ľṹ��ʽ[CH2=C(CH3)-COCl]��������Ϣʹ�Ľṹ��ʽΪ
���ٽ��G�Ľṹ��ʽ[CH2=C(CH3)-COCl]��������Ϣʹ�Ľṹ��ʽΪ ��
��
��D��Ħ������M��6.25��16��100g/mol���ɴ˿�ȷ���������������ԭ�����ֱ�ΪN��C���� ��N��H����
��N��H����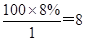 ��N��O����
��N��O����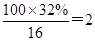 ������ʽΪC5H8O2�����ӵIJ����Ͷ�Ϊ2���������ͼ����Ϣ��ȷ��D�Ľṹ��ʽΪCH2=C(CH3)-COOCH3��������������Ϊ̼̼˫����������
������ʽΪC5H8O2�����ӵIJ����Ͷ�Ϊ2���������ͼ����Ϣ��ȷ��D�Ľṹ��ʽΪCH2=C(CH3)-COOCH3��������������Ϊ̼̼˫����������
�ɸ��ݺ�����Ϣʹ�߷���ҩ��Ľṹ��ʽ��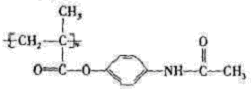 ������ÿ�������к���������������Һ��Ӧ�Ĺ�������1���������ɷ��ǻ��γɵ���������1���ļ����ݴ˱��д���÷�Ӧ����ʽ��
������ÿ�������к���������������Һ��Ӧ�Ĺ�������1���������ɷ��ǻ��γɵ���������1���ļ����ݴ˱��д���÷�Ӧ����ʽ��
�ʸ���D�ķ���ʽΪC5H8O2���䲻���Ͷ�Ϊ2���ٸ�����������ȷ����ͬ���칹����Ҫ����̼̼˫������OOCH����ͬ���칹��ֱ��У�CH2��CH��CH2��CH2��OOCH��CH3��CH��CH��CH2��OOCH����˳���ṹ����CH3��CH2��CH��CH��OOCH����˳���ṹ����CH2��C(CH3)��CH2��OOCH��(CH3)2C��CH��OOCH��CH2��CH��CH(CH3)��OOCH��CH3��CH��C(CH3)��OOCH����˳���ṹ����CH2��C(C2H5)��OOCH����11�֡�
���㣺�����л��ƶϣ���Ӧ���͵��жϣ�����ʽ��ȷ�����л���Ӧ����ʽ���л��ļ������ƶϣ�ͬ���칹���������ļ���ȡ�


 Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����˵������ȷ����
| A����Ȼ��֬������������������ |
| B����ѿ�Ǻ����ǵ�ˮ�������ͬ |
| C����ȩ��֬�Ƿ���ȩ�����۲��� |
| D��ʯ���ѻ����ѽⶼ���Ƶ�ϩ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��12�֣������л�������ת����ϵ����ͼ�����ַ�Ӧ��������ȥ����
��1��A�ĽṹʽΪ ��C�й����ŵ�����Ϊ ��D���ʵ�����Ϊ ��
��2������ȡ����Ӧ���� ������ţ���
��3��A��������G������ʽΪC6H6����G����������ԭ����ͬһƽ�棬G�Ȳ���ʹ���Ը��������Һ��Ӧ��ɫ��Ҳ����ʹ��ˮ��Ӧ��ɫ����G�Ľṹ��ʽΪ ��
��4��H��E��ͬ���칹�壬�ܷ���ˮ�ⷴӦ��H�Ľṹ��ʽ����Ϊ ��
��5����Ӧ�۵Ļ�ѧ����ʽΪ ����Ӧ�ݵĻ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����14�֣�
M��һ������ֱ������Сϸ���ΰ�ҩ�����Ҫ�ɷ֣���ṹʽΪ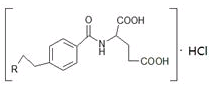 ������������ṹ������RΪ
������������ṹ������RΪ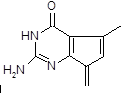 ����M��һ���ϳ�·�����£����ַ�Ӧ�Լ���������ȥ����
����M��һ���ϳ�·�����£����ַ�Ӧ�Լ���������ȥ����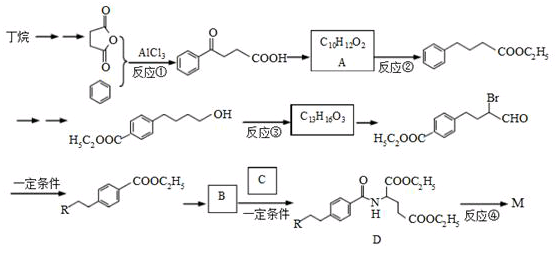
���������գ�
(1)д����Ӧ���͡� ��Ӧ��_________ ��Ӧ��__________
(2)д����Ӧ�Լ��ͷ�Ӧ��������Ӧ��_________ ��Ӧ��__________
(3)д���ṹ��ʽ�� B_____________ C_____________
(4)д��һ����������������A��ͬ���칹��Ľṹ��ʽ��
��1������FeCl3��Һ������ɫ��Ӧ����2���ܷ���������Ӧ����3����������5�ֲ�ͬ��ѧ��������ԭ�ӡ�
(5)�����ȴ��ɵõ�2���ȶ��飬���һ����2���ȶ���ϳ�1,3������ϩ�ĺϳ�·�ߡ�
���ϳ�·�߳��õı�ʾ����Ϊ��A B����
B���� Ŀ����
Ŀ����
(6)��֪��R��CO��NHR����R��CO��OR��Ļ�ѧ�������ơ�
�ӷ�Ӧ�ܿɵó��Ľ�����:_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��15�֣�����ѧ��ѡ��5���л���ѧ������
��֪�л���A��I֮���ת����ϵ����ͼ��ʾ��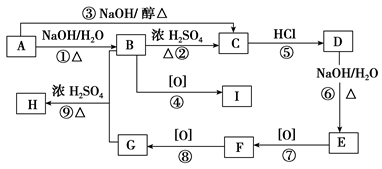
��֪��
����A��D��B��E��I��F��Ϊͬ���칹��
���ڽ�����Cu(OH)2����Һ�ֱ���뵽�л���I��F�У����ȣ�I����������F��Ӧ������ש��ɫ����
����C��ʵ��ʽ����Ȳ��ͬ������Է�������Ϊ104
�� ��E��һ��ͬ���칹����FeCl3�ܷ�����ɫ��Ӧ
����������Ϣ���ش��������⣺
��1��H�� F�к��еĺ������������Ʒֱ�Ϊ �� ��
��2����Ӧ�١�����������ȥ��Ӧ����____________________________��
��3��I�Ľṹ��ʽΪ____________________________��
��4��д��H������������ˮ��Ļ�ѧ����ʽ ��
��5��д��F��������Һ��Ӧ�Ļ�ѧ����ʽ ��
��6����������������G��ͬ���칹����________�֣����б�����ֻ��2��һԪȡ����Ľṹ��ʽΪ ��
�����ڷ����廯����
������NaOH��Һ��Ӧ
���ܷ���������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��18�֣��ȶ���M������ĵ��ߵ��²��ϣ���ҵ�Ͽ����л�����ԭ��A��E�Ƶã���ϳ�·������ͼ��ʾ��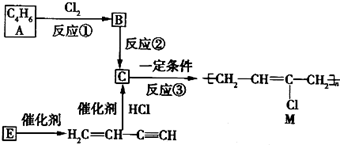
��֪��H2C=CH-C��CH��E���۵õ���
���������գ�
(1)A��������_________________����Ӧ�۵ķ�Ӧ������_________��Ӧ��
(2)д����Ӧ�ڵĻ�ѧ��Ӧ����ʽ�� _______________________________________��
(3)Ϊ�о����ʵķ����ԣ���E���ۡ��ľ۳ɻ�״�����д�����ǵĽṹ��ʽ�� _________��_________ ��������������״��������Լ�Ϊ_________________��
(4)��������A�Ʊ���������PB��ԭ��֮һһl��4-������(BDO)�ĺϳ�·�ߣ�
д��������A�Ʊ�BDO�Ļ�ѧ����ʽ��
��Ӧ��_______________________________________________________________
��Ӧ��_______________________________________________________________
��Ӧ��_______________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ��ͨ���Ҷ������������ðѰ�˾ƥ�������ڸ߾����ϣ��Ƴɻ��ͳ�Ч��˾ƥ�֣����ڹؽ����IJ��ĸ������ƣ����ͳ�Ч��˾ƥ�ֵĽṹ��ʽ���£�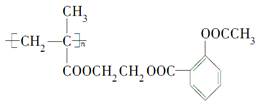
����д���¿հף�
(1)�߷�������ṹ��ʽΪ________��
(2)��˾ƥ�������ڸ߷��������ϵ��л���Ӧ������________________��
(3)���ͳ�Ч��˾ƥ���ڳ�θ�б�Ϊ��˾ƥ�ֵĻ�ѧ����ʽ��______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������һ����������Ѫ�ܼ�����ҩ����ɻ�����E��M��һ�������ºϳɵõ�(���ַ�Ӧ������)��
(1)A������Ϊ________��A��B�ķ�Ӧ����Ϊ________��
(2)D��E�ķ�Ӧ�У�����Ļ�����X������Cu(OH)2��Ӧ������ɫ�����Ļ�ѧ����ʽΪ________��
(3)G��JΪȡ����Ӧ������һ��������еĹ�������________��
(4)L��ͬ���칹��Q�Ƿ����ᣬQ R(C8H7O2Cl)
R(C8H7O2Cl) S
S T��T�ĺ˴Ź�������ֻ������壬Q�Ľṹ��ʽΪ________��R��S�Ļ�ѧ����ʽΪ________��
T��T�ĺ˴Ź�������ֻ������壬Q�Ľṹ��ʽΪ________��R��S�Ļ�ѧ����ʽΪ________��
(5)��ͼ�У������ϳ����߷��ӻ�����ķ������________��
��6����֪��L��M��ԭ��Ϊ��
��
��
��M�Ľṹ��ʽΪ_________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
?1?1 mol��ͪ��? ?�������������¼�1 mol����ת��������ᡣ����Ľṹ��ʽΪ_________________________________________��
?�������������¼�1 mol����ת��������ᡣ����Ľṹ��ʽΪ_________________________________________��
?2?�����������ͬ�����ŵ������ͬ���칹��A�����������£�����ʧˮ����B����A����B�Ļ�ѧ��Ӧ����ʽ��_________________________________
?3?B�ļ������Ծۺϣ��ۺ���Ľṹ��ʽ������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com