
��10�֣�����������ˮ���뵽NaI��Һ�С���ѧ��ʵ������˴��������Һ��ij�о�С���øú����Һ�Ʊ�����NaI��ʵ���������£�
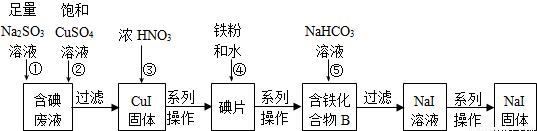
��֪����Ӧ��2I-+2Cu2++SO32-+H2O�T2CuI��+SO42-+2H+
�ش��������⣺
��1�����������Һ�����ӷ���ʽΪ________________________________��
��2������I2��Na2SO3��Һ��Ӧ�����ӷ���ʽΪ______________________________��
��3������CuI������������Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ_____________________��
��4��������B�к�����Ԫ�أ���Ԫ������һ��Ԫ�����ʵ���֮��Ϊ3��8������B�Ļ�ѧʽΪ___________________��
��5����Ӧ�������ɺ�ɫ�������ɫ���壬��ɫ������׳�Ϊ��������������ݵĻ�ѧ����ʽΪ___________________��
 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꽭��ʡ��һ�ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
NA��ʾ�����ӵ�������ֵ������˵����ȷ����
A��0��5mol O3��������16g B��32gO2�к��е�����������Ϊ2NA
C��1gH2�к��еĵ�����ΪNA D��0��5NA��ͭԭ�ӵ�����Ϊ64g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�������ʡ������ѧ�����в��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����йػ�ѧ�����ʾ��ȷ����
A����������ķ���ʽ��SiO2
B������״̬��������صĵ��뷽��ʽ��KHSO4 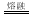 K++HSO4-
K++HSO4-
C�����ĵ���ʽ��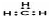
D. HClO�Ľṹʽ��H��Cl��O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016��㶫ʡ�����и�����ѧ�ڵڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
������ڻ�ѧѧ�Ƶķ�չ������Ҫ�����ã����з������������
A�����ݴ������е�Ԫ����ɣ����������Ϊ���ʺͻ�����
B��������Һ��������ǿ����������ʷ�Ϊǿ����ʡ��������
C�������Ƿ���ж����ЧӦ������ɢϵ��Ϊ��Һ����Һ�ͽ���
D�����ݷ�Ӧ�е������仯������ѧ��Ӧ��Ϊ�����ϡ��ֽ⡢���ֽ⡢�û�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�걱����ѧ������ѧ��У��һ�ϳ鿼��ѧ�Ծ��������棩 ���ͣ������
����Ϊ��ѧ��ѧ�г��������ʣ��� Cu ��NaCl ���� �� NaHSO4 ���� �� SO3 ��ϡ H2SO4 �� C2H5OH �� CaCO3 ���� �� BaSO4 ���� ������ NaOH �� ��ˮ �밴���з�����ش����⡣����д���ţ�
��1���ܵ������_________________________
��2������ǿ����ʵ���_________________________
��3����д��������ˮ��Һ�ĵ��뷽��ʽ��
NaHSO4=_________________________
NaHCO3=_________________________
��4����дNaOH��Һ������ Ca��HCO3��2 ��Һ��Ӧ�����ӷ���ʽ��_________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016��ӱ�ʡ������ѧ�����в��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��NA���������ӵ���������ֵ������˵����ȷ����
A��10 mL 20 mol•L-1Ũ����������п��Ӧ��ת�Ƶ�����Ϊ0.2NA
B��0.1 mol24Mg18O������������������Ϊ2.0 NA
C���ڱ�״���£�2.8g N2��2.24L CO������������Ϊ1.4NA
D��1 L 1 mol•L-1��NaClO��Һ�к���ClO-����ĿΪNA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�콭��ʡ������ѧ��9��ѧ����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ͼ��ʾװ�ý�������ʵ�飺��������Һ������У�Ԥ���������ʵ���������
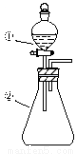
ѡ�� | �������� | �������� | Ԥ����е����� |
A | ϡ���� | ̼�������������ƵĻ����Һ | ������������ |
B | Ũ���� | ��ɰֽ��ĥ�������� | ��������ɫ���� |
C | �Ȼ�����Һ | Ũ����������Һ | ����������ɫ���� |
D | ����������Һ | �������������Һ | ��Һ����ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ���Ĵ�ʡ�߶�10���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��һ���̶��ݻ����ܱ������У�����3L X(��)��2L Y(��)����һ�������·������з�Ӧ��4X(g) �� 3Y(g)  2Q(g) �� nR(g)���ﵽƽ����������¶Ȳ��䣬��������ѹǿ��ԭ������5%��X��Ũ�ȼ�С1/3����÷�Ӧ����ʽ�е�nֵ��( )
2Q(g) �� nR(g)���ﵽƽ����������¶Ȳ��䣬��������ѹǿ��ԭ������5%��X��Ũ�ȼ�С1/3����÷�Ӧ����ʽ�е�nֵ��( )
A��3 B��4 C��5 D��6
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ������ʦ���и�һ��10���¿���ѧ�Ծ� �������棩 ���ͣ�ѡ����
M��NO3��2�ȷֽ⻯ѧ����ʽΪ��2M��NO3��2 2MO+4NO2��+O2��������29.6g M��NO3��2ʹ����ȫ�ֽ⣬�ڱ�״�����ռ�11200mL�����壬��ôM��Ħ��������
2MO+4NO2��+O2��������29.6g M��NO3��2ʹ����ȫ�ֽ⣬�ڱ�״�����ռ�11200mL�����壬��ôM��Ħ��������
A��64g/mol B��24g/mol C��65g/mol D��40g/mol
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com