
ij��ˮ�������������е�5�֣�����ˮ�ĵ��뼰���ӵ�ˮ�⣩��K+��Cu2+��Al3+��Fe2+��Cl����CO32����NO3����SO42������ø������ӵ����ʵ���Ũ����ȡ�Ϊ̽����ˮ����ɣ�ijͬѧ����������ʵ�飺
���ò�˿պȡ������Һ���ڻ��������գ�����ɫ�ܲ����۲�����ɫ���档
����ȡ��Һ��������ϡ���ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ����ʱ��Һ��Ȼ���壬����Һ������������䡣
����ȡ��Һ����BaCl2��Һ���а�ɫ�������ɡ�
��������ʵ�飬�����Ʋ���ȷ���ǣ� ��
A����Һ����ȷ��Al3+�Ĵ������ B��ԭ��Һ�в���������Ϊ��K+��Al3+��CO32��
C��������п���ȷ��Fe2+��NO3���Ĵ��� D��������й���2�ֱ��γ���
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)(2013���˴�ģ��)�ٺ�0.4 mol Al3+��Al2(SO4)3��������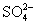 �����ʵ�����______________��
�����ʵ�����______________��
��2.3 g Na�к����ӵ����ʵ���Ϊ��������mol,�ڸ�����ˮ��Ӧ��ʧȥ���ӵ����ʵ���Ϊ��������mol��
(2)(2013������ģ��)��48 g 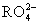 ��,�����������������������6.02��1023��,��RԪ�ص�Ħ������Ϊ����������
��,�����������������������6.02��1023��,��RԪ�ص�Ħ������Ϊ����������
����һ���ƿ������ΪM1 g,��ƿ���������������ΪM2 g;����ͬ״����,���ij�ij����A��,������ΪM3 g,��A����Է�������Ϊ����������
(3)(2013�� ����ʦ����ģ��)һ������������������ȼ��,���û������
100 mL 3.00 mol��L-1��NaOH��Һ(�ܶ�Ϊ1.12 g��mL-1)ǡ����ȫ����,�����Һ�к���NaClO�����ʵ���Ϊ0.050 0 mol��
��ԭNaOH��Һ����������Ϊ___________________________;
��������Һ��Cl-�����ʵ���Ϊ_________________________mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���б仯��������Ҫ���뻹ԭ������ �� ��
A��KClO3 ��KCl B��NaCl��AgCl C��H����H2 D��C��CO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
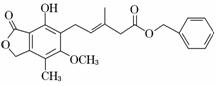
�й���ͼ��ʾ�������˵������ȷ���� (�� ��)
A���ȿ�����Br2��CCl4��Һ�����ӳɷ�Ӧ���ֿ����ڹ�������Br2����ȡ����Ӧ
B��1 mol�û�������������3 mol NaOH��Ӧ
C���ȿ��Դ����⣬�ֿ���ʹ����KMnO4��Һ��ɫ
D���ȿ�����FeCl3��Һ������ɫ��Ӧ���ֿ�����NaHCO3��Һ��Ӧ�ų�CO2����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧ�о���ѧϰС��Ե������Һ�����µĹ����ܽ�(���ڳ�����)��������ȷ���ǣ� ��
�ٳ����£�pH=1��ǿ����Һ����ˮϡ�ͺ���Һ������Ũ��һ������
��pH=2�������pH=1�����ᣬc(H+)֮��Ϊ2:1
��pH��ȵ�������Һ��a��CH3COONa��b��C6H5ONa��c��NaHCO3��d��NaOH������Һ���ʵ���Ũ����С����˳��Ϊd��b��c��a
��NH4HSO4��Һ�еμ�NaOH��Һ����ҺpH=7����c(Na+)=2c(SO42-)
����֪�������ƽ�ⳣ��ΪKa�������ˮ�ⳣ��ΪKh��ˮ�����ӻ�ΪKw�������߹�ϵΪKa��Kh=Kw
�ס�������Һ����ǿ����ʣ���֪����Һ��pH������ҺpH����������ס�������Һ�������ϣ����ҺpH���ܵ���7
A���ۢݢ� B���ۢܢ� C���ܢݢ� D���٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ˮú�����Ƽ״��������̿�ͼ����
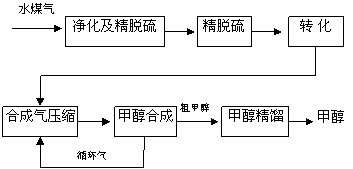
(ע����ȥˮ�������ˮú����55��59%��H2��15��18%��CO��11��13%��CO2��������H2S��CH4����ȥH2S�ɲ��ô���Ǵ�ת����������CH4ת����CO���õ�CO��CO2��H2�Ļ�����壬������ĺϳɼ״�ԭ���������ɽ��м״��ϳ�)
��1����ˮú������Ҫ��ѧ��Ӧ����ʽΪ��C��s��+H2O��g�� CO��g��+H2��g�����˷�Ӧ�����ȷ�Ӧ���� �˷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ ��
CO��g��+H2��g�����˷�Ӧ�����ȷ�Ӧ���� �˷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ ��
�����������̼��ƽ��ת���ʵĴ�ʩ�� ��
A������C��s��
B������H2O��g��
C�������¶�
D������ѹǿ
��2����CH4ת����CO����ҵ�ϳ����ô�ת���������䷴Ӧԭ��Ϊ��
CH4 (g)+3/2O2 (g) CO (g)+2H2O (g) ��H=-519KJ/mol����ҵ��Ҫѡ����ʵĴ������ֱ��X��Y��Z���ִ�����������ʵ�飨����������ͬ��
CO (g)+2H2O (g) ��H=-519KJ/mol����ҵ��Ҫѡ����ʵĴ������ֱ��X��Y��Z���ִ�����������ʵ�飨����������ͬ��
�� X��T1��ʱ��Ч����ߣ���ʹ����Ӧ���ʼӿ�Լ3��105����
�� Y��T2��ʱ��Ч����ߣ���ʹ����Ӧ���ʼӿ�Լ3��105����
�� Z��T3��ʱ��Ч����ߣ���ʹ�淴Ӧ���ʼӿ�Լ1��106����
��֪��T1��T2��T3������������Ϣ������Ϊ��������Ӧ��ѡ������˴����� ���X����Y����Z������ѡ��������� ��
��3���ϳ�����ѹ�����º����10m3�״��ϳ������ڴ��������£����м״��ϳɣ���Ҫ��Ӧ�ǣ�2H2(g) + CO(g)  CH3OH(g) ��H1
CH3OH(g) ��H1
��֪��CO(g)��1/2O2(g)��CO2(g) ��H����283 kJ��mol��1
CH3OH(l)��3/2O2(g)��CO2(g) ��2H2O(l) ��H����725kJ��mol��1
H2O(l)= H2(g)+ 1/2O2(g) ��H��+285.8 kJ��mol��1
��������Ӧ����H1= kJ��mol��1��
��T4���´˷�Ӧ��ƽ�ⳣ��Ϊ160�����¶��£����ܱ������м���CO��H2����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
| ���� | H2 | CO | CH3OH |
| Ũ��/��mol��L��1�� | 0.2 | 0.1 | 0.4 |
�Ƚϴ�ʱ�����淴Ӧ���ʵĴ�С��v�� v�� ���>������<������)��
������������ʵ�����CO��H2����T5�淴Ӧ��10 min��ﵽƽ�⣬��ʱc(H2)��0.4 mol��L��1�� c(CO)��0.7 mol��L��1�����ʱ���ڷ�Ӧ����v(CH3OH) �� mol��(L��min)��1��
��4�����������У��ϳ���Ҫ����ѭ������Ŀ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʵ�����У����г�ȥ���ʵķ�����ȷ����( )
�������л���ϩ��ͨ��������һ�������·�Ӧ��ʹ��ϩת��Ϊ���飻
�ڳ�ȥ��������������������ñ���̼������Һϴ�ӣ���Һ���������
�۳�ȥCO2��������SO2������ͨ��ʢ����̼������Һ��ϴ��ƿ��
�ܳ�ȥ�Ҵ��������������������ʯ�ң�����
���屽�л����壬����KI��Һ������������ȡ���壻
���������л���ŨHNO3��ŨH2SO4�����䵹��NaOH��Һ�У����ú��ٹ��ˡ�
A���٢ڢۢ� B���ڢ� C���ڢۢ� D���ڢۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ���������ȷ����( )
A����ȡ�屽������м����ˮ������ϼ���
B��ʵ������ȡ���������ȼ���Ũ���ᣬ�ټӱ���������Ũ����
C������ױ��ͱ�����ױ��ͱ��зֱ��������KMnO4��Һ�����۲��Ƿ���ɫ
D��ͨ������ˮ�м����Ҵ�����ȡ��ˮ�е���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ס��ҡ����������������У��ס��ҡ�����������ͬ��ij��Ԫ�أ�����֮���������ת����ϵ��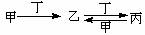 �������й����ʵ��ƶϲ���ȷ����( )
�������й����ʵ��ƶϲ���ȷ����( )
A.����Ϊ��̿��������O2 B.����ΪSO2�������ǰ�ˮ
C.����ΪFe������������ D.����ΪNaOH��Һ��������CO2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com