

·ÖĪö øł¾ŻĆ¾ŗĶĀĮ»ÆѧŠŌÖŹµÄ²īŅģ£ŗĀĮæÉŅŌÓė¼ī·“Ó¦£¬ČĆĆ¾ŗĶĀĮ·ÖĄėµÄ·½·ØŹĒŹ¹ĀĮČÜÓŚĒāŃõ»ÆÄĘČÜŅŗ£¬¹ĢĢåBĆ¾²»Čܽā£¬¹żĀĖ·ÖĄė£¬ŌŁ½«ČÜŅŗAĘ«ĀĮĖįÄĘÓėČõĖį·“Ó¦±äĪŖ¹ĢĢåCĒāŃõ»ÆĀĮ£¬ĒāŃõ»ÆĀĮ¼ÓČČ·Ö½āµĆµ½ČżŃõ»Æ¶žĀĮ£®
½ā“š ½ā£ŗøł¾ŻĆ¾ŗĶĀĮ»ÆѧŠŌÖŹµÄ²īŅģ£ŗĀĮæÉŅŌÓė¼ī·“Ó¦£¬ČĆĆ¾ŗĶĀĮ·ÖĄėµÄ·½·ØŹĒŹ¹ĀĮČÜÓŚĒāŃõ»ÆÄĘČÜŅŗ£¬¹ĢĢåBĆ¾²»Čܽā£¬¹żĀĖ·ÖĄė£¬ŌŁ½«ČÜŅŗAĘ«ĀĮĖįÄĘÓėČõĖį·“Ó¦±äĪŖ¹ĢĢåCĒāŃõ»ÆĀĮ£¬ĒāŃõ»ÆĀĮ¼ÓČČ·Ö½āµĆµ½ČżŃõ»Æ¶žĀĮ£®
£Ø1£©ĀĮÓėĒāŃõ»ÆÄĘ·“Ӧɜ³ÉĘ«ĀĮĖįÄĘŗĶĒāĘų£¬Ąė×Ó·½³ĢŹ½2Al+2OH-+2H2OØT2AlO2-+3H2”ü£¬¹Ź“š°øĪŖ£ŗ2Al+2OH-+2H2OØT2AlO2-+3H2”ü£»
£Ø2£©¹ĢĢåŗĶŅŗĢå·ÖĄėÓĆ¹żĀĖ£¬¹Ź“š°øĪŖ£ŗ¹żĀĖ£»
£Ø3£©Ę«ĀĮĖįÄĘÓėĖį·“Ó¦æɵƵ½ĒāŃõ»ÆĀĮ£¬ĒāŃõ»ÆĀĮæÉČÜÓŚŃĪĖį£¬¹ŹÖ»ÄÜÓƶžŃõ»ÆĢ¼£¬¹Ź“š°øĪŖ£ŗD£»
£Ø4£©Ļ“µÓ³Įµķ³żµōæÉČÜŠŌŌÓÖŹ£¬¹Ź“š°øĪŖ£ŗĻ“µÓ³Įµķ£»
£Ø5£©¼ÓČČĒāŃõ»ÆĀĮæɵƵ½ČżŃõ»Æ¶žĀĮ£¬»Æѧ·½³ĢŹ½ĪŖ2Al£ØOH£©3ØTAl2O3+3H2O£¬¹Ź“š°øĪŖ£ŗ¼ÓČČ£»2Al£ØOH£©3ØTAl2O3+3H2O£®
µćĘĄ ±¾Ģāæ¼²éĪļÖŹµÄ·ÖĄė”¢Ģį“棬²ąÖŲӌѧɜµÄ·ÖĪöÄÜĮ¦”¢ŹµŃéÄÜĮ¦ŗĶĘĄ¼ŪÄÜĮ¦µÄ漲飬ÄŃ¶Č²»“ó£¬×¢Ņā°ŃĪÕĪļÖŹµÄŠŌÖŹµÄŅģĶ¬£¬×¢Ņā³żŌÓŹ±²»ÄÜŅżČėŠĀµÄŌÓÖŹ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ijĶ¬Ń§ŅŌøŹÕįŌüĪŖŌĮĻÓĆĖ®½āŅ»Ńõ»ÆŅ»Ė®½āŃ»·½ųŠŠÖĘČ”²ŻĖį²¢Ģ½¾æ²ā¶Ø²ŻĖį¾§Ģå£ØH2C2O4•xH2O£©µÄijŠ©ŠŌÖŹ£®Ķعż²éŌÄ׏ĮĻæÉÖŖ£ŗ²ŻĖįŅ×ČÜÓŚĖ®£¬ŹĒŅ»ÖÖÖŲŅŖµÄ»Æ¹¤ŌĮĻ£¬¹ć·ŗÓĆÓŚŅ©ĪļÉś²ś”¢øß·Ö×ÓŗĻ³ÉµČ¹¤Ņµ£¬²ŻĖį¾§ĢåŹÜČȵ½100”ꏱŹ§Č„½į¾§Ė®£¬³ÉĪŖĪŽĖ®²ŻĖį£®157”ꏱ“óĮæÉż»Ŗ£¬²¢æŖŹ¼·Ö½ā£»²ŻĖįÕōĘųŌŚµĶĪĀĻĀæÉĄäÄżĪŖ¹ĢĢ壻²ŻĖįøĘ²»ČÜÓŚĖ®£¬²ŻĖįÕōĘųÄÜŹ¹³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē£®
ijĶ¬Ń§ŅŌøŹÕįŌüĪŖŌĮĻÓĆĖ®½āŅ»Ńõ»ÆŅ»Ė®½āŃ»·½ųŠŠÖĘČ”²ŻĖį²¢Ģ½¾æ²ā¶Ø²ŻĖį¾§Ģå£ØH2C2O4•xH2O£©µÄijŠ©ŠŌÖŹ£®Ķعż²éŌÄ׏ĮĻæÉÖŖ£ŗ²ŻĖįŅ×ČÜÓŚĖ®£¬ŹĒŅ»ÖÖÖŲŅŖµÄ»Æ¹¤ŌĮĻ£¬¹ć·ŗÓĆÓŚŅ©ĪļÉś²ś”¢øß·Ö×ÓŗĻ³ÉµČ¹¤Ņµ£¬²ŻĖį¾§ĢåŹÜČȵ½100”ꏱŹ§Č„½į¾§Ė®£¬³ÉĪŖĪŽĖ®²ŻĖį£®157”ꏱ“óĮæÉż»Ŗ£¬²¢æŖŹ¼·Ö½ā£»²ŻĖįÕōĘųŌŚµĶĪĀĻĀæÉĄäÄżĪŖ¹ĢĢ壻²ŻĖįøĘ²»ČÜÓŚĖ®£¬²ŻĖįÕōĘųÄÜŹ¹³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē£®

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 Cl2ŗĶH2O2ŹĒøßÖŠ½×¶Ī×ī³£¼ūµÄĮ½ÖÖŃõ»Æ¼Į£¬¾²éŌÄ׏ĮĻCl2Ńõ»ÆÄÜĮ¦ĒæÓŚH2O2£¬Äܽ«H2O2Ńõ»Æ£®ĪŖĮĖŃéÖ¤øĆ½įĀŪ£¬Ń§ÉśÉč¼ĘĮĖČēĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆ½ųŠŠŹµŃé£Ø¼Š³Ö×°ÖĆĀŌČ„£©£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
Cl2ŗĶH2O2ŹĒøßÖŠ½×¶Ī×ī³£¼ūµÄĮ½ÖÖŃõ»Æ¼Į£¬¾²éŌÄ׏ĮĻCl2Ńõ»ÆÄÜĮ¦ĒæÓŚH2O2£¬Äܽ«H2O2Ńõ»Æ£®ĪŖĮĖŃéÖ¤øĆ½įĀŪ£¬Ń§ÉśÉč¼ĘĮĖČēĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆ½ųŠŠŹµŃé£Ø¼Š³Ö×°ÖĆĀŌČ„£©£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ HCl+HClO£»2HClO$\frac{\underline{\;¹āÕÕ\;}}{\;}$2HCl+O2”ü£®£ØÓĆ·½³ĢŹ½»Ų“š£©£®¶ŌÓŚÖŹŅÉæÉŅŌ²ÉÓƶŌ±ČŹµŃé½ā¾ö£®
HCl+HClO£»2HClO$\frac{\underline{\;¹āÕÕ\;}}{\;}$2HCl+O2”ü£®£ØÓĆ·½³ĢŹ½»Ų“š£©£®¶ŌÓŚÖŹŅÉæÉŅŌ²ÉÓƶŌ±ČŹµŃé½ā¾ö£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | CH3CH=C£ØCH3£© CH3 3-¼×»ł-2-¶”Ļ© | |
| B£® | CH3CH£ØCH3£©CH£ØCl£©CH3 3-¼×»ł-2-Āȶ”Ķé | |
| C£® | CH3CH£ØOH£©CH2CH3 2-ōĒ»ł¶”“¼ | |
| D£® | CH3CH£ØC2H5£©CH2CH2CH3 2-ŅŅ»łĪģĶé |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | CH2=CH2+H-OH$\frac{\underline{“߻ƼĮ}}{”÷}$CH3-CH2-OH | |
| B£® | CH2=CH-CH=CH2+2H2$\frac{\underline{\;“߻ƼĮ\;}}{\;}$CH3-CH2-CH2-CH3 | |
| C£® |  +H2$\stackrel{“߻ƼĮ}{”ś}$CH3-CH2-OH +H2$\stackrel{“߻ƼĮ}{”ś}$CH3-CH2-OH | |
| D£® | CH3-CH3+2Cl2$\stackrel{¹āÕÕ}{”ś}$CH2Cl-CH2Cl+2HCl |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 2g H2ŗ¬ÓŠµÄĒāŌ×ÓŹżÄæĪŖNA | |
| B£® | ³£ĪĀ³£Ń¹ĻĀ£¬22.4 LO2ŗ¬ÓŠµÄ·Ö×ÓŹżÄæĪŖNA | |
| C£® | 1 molZn×Ŗ±äĪŖZn2+Ź§Č„µÄµē×ÓŹżÄæĪŖNA | |
| D£® | 1 L 1mol•L-1KOHČÜŅŗÖŠŗ¬ÓŠµÄ¼ŲĄė×ÓŹżÄæĪŖNA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £¬Ō¤²āøĆ·¼ĻćĢžÄÜ£ØĢī”°ÄÜ”±»ņ”°²»ÄÜ”±£©·¢ÉśøĆĄą·“Ó¦£®
£¬Ō¤²āøĆ·¼ĻćĢžÄÜ£ØĢī”°ÄÜ”±»ņ”°²»ÄÜ”±£©·¢ÉśøĆĄą·“Ó¦£®
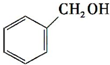 ””c£®
””c£®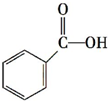
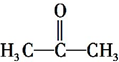
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com