
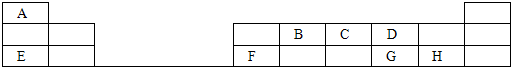
ÓÉA”¢D”¢E”¢GĖÄÖÖŌŖĖŲ×é³ÉµÄ»ÆŗĻĪļµÄ·Ö×ÓŹ½ĪŖ![]() £®ŅŃÖŖ£ŗ
£®ŅŃÖŖ£ŗ
(1)![]() ĪŖÕūŹż£¬ĒŅ
ĪŖÕūŹż£¬ĒŅ![]() £®
£®
(2)Č”8.74gøĆ»ÆŗĻĪļČÜÓŚĖ®£¬¼ÓČėĖįŗĶĒæŃõ»Æ¼Į£®»ÆŗĻĪļÖŠµÄE”¢GŌŖĖŲĶźČ«×Ŗ»Æ³ÉĘųĢ¬»ÆŗĻĪļ![]() 2.688L(±ź×¼×“æöĻĀ)£¬ĘäĆܶČĪŖ1.965g/L£¬
2.688L(±ź×¼×“æöĻĀ)£¬ĘäĆܶČĪŖ1.965g/L£¬![]() ÄÜŹ¹³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē£®
ÄÜŹ¹³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē£®
(3)·¢ÉśÉĻŹö·“Ó¦ŗó£¬A”¢DŅŌŃōĄė×Ó“ęŌŚÓŚČÜŅŗÖŠ£®ĶłČÜŅŗĄļ¼ÓČė¹żĮæµÄĢś·Ū£¬ĘäÖŠŹ¹Ąė×ÓČ«²æ»¹ŌĖłĻūŗĵÄĢś·ŪÖŹĮæĪŖ0.56g£®
(4)»ÆŗĻĪļÖŠDŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ12.8£„£®
ŹŌĶعż¼ĘĖćŗĶĶĘĄķČ·¶ØE”¢G”¢D”¢Aø÷ŹĒŹ²Ć“ŌŖĖŲ£¬Ēó³ö![]() µÄÖµ£¬²¢Š“³öøĆ»ÆŗĻĪļµÄ»ÆѧŹ½£®
µÄÖµ£¬²¢Š“³öøĆ»ÆŗĻĪļµÄ»ÆѧŹ½£®
|
“š°ø£ŗ (1)EŹĒĢ¼£¬GŹĒŃõ£® (2) (3)AŹĒ¼Ų£¬øĆ»ÆŗĻĪļ»ÆѧŹ½ĪŖ ŅĄ¾ŻŹµŃ鏿¾Ż£¬Ķعż¼ĘĖć£¬·ÖĪöŗĶĶĘĄķ£¬Č·¶ØĪ“ÖŖĪļµÄ»ÆѧŹ½£¬ŹĒ¶ØĮæŃŠ¾æ»ÆѧµÄŅ»øöÖŲŅŖæĪĢā£®ŌŚ±¾ĢāÖŠ£¬
ÓÖ“Ó ¶žŃõ»ÆĢ¼µÄĪļÖŹµÄĮæŹĒ£ŗ DĄė×ÓŹĒ¾ßÓŠŃõ»ÆŠŌµÄ½šŹōĄė×Ó£¬ÓĆ¹żĮæĢś·Ū»¹ŌDĄė×Ó£¬Fe±»Ńõ»ÆĪŖ
ČōĆæøöDĄė×ӵƵ½1øöµē×Ó£¬Ōņ±»»¹ŌµÄDµÄĪļÖŹµÄĮæĪŖ0.02mol£®ŌŚøĆ»ÆŗĻĪļÖŠDÓė(
”ß ”ą DŌŖĖŲµÄĻą¶ŌŌ×ÓÖŹĮæĪŖ£ŗ
ÓÉ“ĖæÉÖŖDŹĒĢś£¬DĄė×ÓŹĒ
AŌŖĖŲµÄĻą¶ŌŌ×ÓÖŹĮæĪŖ£ŗ
ÕāŃł£¬Ķعż¼ĘĖć·ÖĪöøĆĪ“ÖŖĪļµÄ»ÆѧŹ½ĪŖ (1)EŹĒĢ¼£¬GŹĒŃõ£® (2) (3)AŹĒ¼Ų£¬øĆ»ÆŗĻĪļ»ÆѧŹ½ĪŖ |
|
ŌŚFe·Ū¹żĮæĢõ¼žĻĀ£¬FeÖ»Äܱ»Ńõ»ÆĪŖ |


 ŹĄ¼Ķ°ŁĶØĘŚÄ©½š¾ķĻµĮŠ“š°ø
ŹĄ¼Ķ°ŁĶØĘŚÄ©½š¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ





²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗĪļĄķ½ĢŃŠŹŅ ĢāŠĶ£ŗ022
ŅŃÖŖ£ŗ£Ø1£©x”¢y”¢zĪŖÕūŹż£¬ĒŅČżÕßÖ®ŗĶµČÓŚ7”£
£Ø2£©Č”8.74gøĆ»ÆŗĻĪļČÜÓŚĖ®£¬¼ÓČėĖįŗĶĒæŃõ»Æ¼Į£¬Ō»ÆŗĻĪļÖŠE”¢GĮ½ÖÖŌŖĖŲĶźČ«×Ŗ»ÆĪŖĆܶČĪŖ1.977g”ĮL-1£¬Ģå»żĪŖ2.688L£Ø±źæö£©µÄĘųĢåEG2£¬øĆĘųĢåæÉŅŌŹ¹³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē”£
£Ø3£©·¢ÉśÉĻŹö·“Ó¦ŗó£¬A”¢DµÄŃōĄė×ÓŠĪŹ½“ęŌŚÓŚČÜŅŗÖŠ£¬ĻņČÜŅŗÖŠ¼ÓČė¹żĮæĢś·Ū£¬ĘäÖŠDĄė×ÓČ«²æ»¹ŌĖłĻūŗĵÄĢś·ŪĪŖ0.56g”£
£Ø4£©»ÆŗĻĪļÖŠDŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ12.81%”£
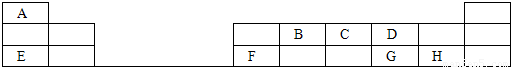
ĒėĶعż¼ĘĖćŗĶĶĘĄķČ·¶ØE”¢D”¢G”¢Aø÷ĪŖŗĪÖÖŌŖĖŲŅŌ¼°ĖüĆĒĖł×é³ÉµÄÉĻŹö»ÆŗĻĪļµÄ»ÆѧŹ½”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(1)x”¢y”¢zĪŖÕūŹż£¬ĒŅx+y+z=7”£
(2)Ȕ8.
(3)·¢ÉśÉĻŹö·“Ó¦ŗó£¬A”¢DŅŌŃōĄė×Ó“ęŌŚÓŚČÜŅŗÖŠ”£ĶłČÜŅŗĄļ¼ÓČė¹żĮæµÄĢś·Ū£¬ĘäÖŠŹ¹DĄė×ÓČ«²æ»¹ŌĖłĻūŗĵÄĢś·ŪÖŹĮæĪŖ0.
(4)»ÆŗĻĪļÖŠDŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ12.8£„”£
ŹŌĶعż¼ĘĖćŗĶĶĘĄķČ·¶ØE”¢G”¢D”¢Aø÷ŹĒŹ²Ć“ŌŖĖŲ£¬Ēó³öx”¢y”¢zµÄÖµ£¬²¢Š“³öøĆ»ÆŗĻĪļµÄ·Ö×ÓŹ½”£
½ā£ŗ(1)Č·¶ØEŗĶG£ŗEŹĒ_______________£»GŹĒ________________”£
(2)Č·¶Øx”¢y”¢zŗĶD£ŗ
x=____________£¬y=____________£¬z=____________£»DŹĒ____________”£
(3)Č·¶ØA²¢Š“³ö»ÆŗĻĪļµÄ·Ö×ÓŹ½£ŗ
AŹĒ____________£»øĆ»ÆŗĻĪļµÄ·Ö×ÓŹ½ŹĒ____________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğŗžÄĻŹ”ÉŪŃōŹŠÉŪŃōĻŲŹÆĘėѧŠ£ø߶ž£ØĻĀ£©ĘŚÄ©»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com