
��������������Ҫ�ɷ�Fe 2O3��Fe 3O4��FeO��SiO2�ȣ��ǹ�ҵ��������ķ��������������������Ʊ�����Ȳ�Ʒ����������ͼ��ʾ ��
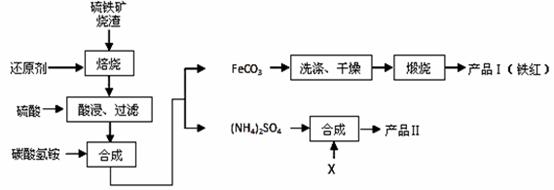
�ش��������⣺
��1��������ʱ��ԭ��̼ȼ�ղ����������ܽ����Ķ��������ﻹԭ�������ʣ����������Fe2O3��Ӧ�Ļ�ѧ����ʽΪ ��
��2�����ʱ��һ�㲻����20 min�����ڿ��������ʱ���������Һ��Fe2+�������½�����ԭ�������ӷ���ʽ��ʾΪ ��
��3����̼����狀ϳ�ʱ��Ӧ�¶�һ���������35�����£���Ŀ���� ��
��4������������FeCO3���ɲ�Ʒ��Ļ�ѧ��Ӧ����ʽΪ ��
��5�������Ʒ��K2SO4�����Ƿ����Ȼ������ʵ�ʵ������ǣ�ȡ������Ʒ�����Թ��������Һ�� ��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijѧϰС���������ʵ�飬̽����ѧ��Ӧ
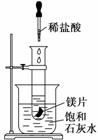
�е���ЧӦ�����Թܷ���ʢ��25��ı���ʯ��ˮ���ձ��У��Թ��п�ʼ���뼸С��þƬ�����õι������еμ�5 mL���ᡣ�Իش��������⣺
(1) ʵ���й۲쵽��������________________________________________________
________________________________________________________________________
________________________________________________________________________��
(2)�������������ԭ����_________________________________________________��
(3)д���йط�Ӧ�����ӷ���ʽ��___________________________________________��
(4)��ʵ����֪��MgCl2��H2��������______(����ڡ�����С�ڡ����ڡ�)þƬ���������������
(5)����������С�25��ı���ʯ��ˮ�����ɡ�20��̼�����ϡ�����ʵ��̽����ʵ���й۲쵽����һ������______________________________________________________
________________________________________________________________________
______________________����ԭ����____________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧΪ������Һ���Ƿ��г��������������ӣ���������ͼ��ʾ��ʵ����������м�������в�����������ʹʪ��ĺ�ɫʯ����ֽ�������ɸ�ʵ���ܵõ�����ȷ������
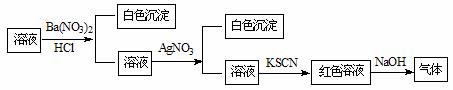
A��ԭ��Һ��һ������SO42������ B��ԭ��Һ��һ������NH4������
C��ԭ��Һ��һ������Cl������ D��ԭ��Һ��һ������Fe3������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
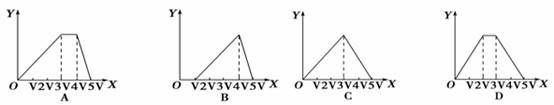
�����ʵ�����ȵ������������������ˮ�γɵĻ����Һ����μ�������������Һֱ�����������б�ʾ����������Һ����������X������Һ�г���������Y���Ĺ�ϵʾ��ͼ����ȷ���ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ơ�þ������0.3mol�ֱ����100mL1mol/L�������У�ͬ��ͬѹ�²���������������Ϊ
A��1��2��3 B��6��3��2 C��3��1��1 D��1��1��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
1.52gͭþ�Ͻ���ȫ�ܽ���50mL��
��Ϊ1.40g·mL-1����������Ϊ63%��Ũ�����У��õ�NO2��N2O4�Ļ������1120mL(��״��)����Ӧ�����Һ�м���1.0mol·L-1NaOH��Һ������������ȫ������ʱ���õ�2.54g������
��1���úϽ���ͭ��þ�����ʵ���֮���� ��
��2��NO2��N2O4�Ļ�������У�NO2����������� ��
��3���õ�2.54 g����ʱ������NaOH��Һ������� mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijУ�о���ѧϰС��ͬѧ��ʵ��ʱ��SO2ͨ��װ��1mol/LBaCl2��Һ���Թ��У������˰�ɫ�����
��1��ʵ��С���ͬѧ���ݳ����ܽ�ƽ��ԭ�������˹������ϵ�������ݣ����������Ƶ��ó��������¼�ʹ��SO2ͨ��1mol/L BaCl2��Һ�������ͣ�Ҳ����������BaSO3�������ʰ�ɫ������ֻ����BaSO4������Ϊ�����ĵ�����Ϊ�� ��
��2����������ͨ���Ȼ�����Һ�������ϲ�������������ʵ���ܵ���������Һ��������Ӱ��ܿ���ܹ۲쵽��������Ϊ�˱������������ij��ѧС�����������ʵ��װ�ã�ʵ������������£�
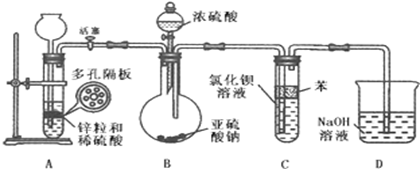
�������Լ����ú���װ��A�еĶ�����Ϸ���п����ͨ�� ȫƷ��ѧ���� �ú��벻�ˣ�ע��ϡ���ᣮ���������������������뵽����װ�ã�
��D�����ݾ���ð�����鴿ȫƷ��ѧ���� �ú��벻�ˣ���رջ�������װ��D�ĵ��ܲ����ձ��С�
��ͨ��װ��B�� ȫƷ��ѧ���� �ú��벻�ˣ��μ�Ũ���ᣬ������������뵽BaCl2��Һ�У���Һ���ֳ��塣
�ܴ������������������������װ��һ��ʱ�䡣
�ݽ�ʢ��BaCl2��Һ���Թܴ�װ����ȡ������ȥ��Ƥ���ý�ͷ�ι����뵽�����·��μ�˫��ˮ���漴���ְ�ɫ���ǣ��μ�ϡ���Ტ����ɫ���Dz���ʧ��
��жװ�ã���ϴ����������ʣ��ҩƷ��
��ش���������
��2��ʵ�鲽��ٺ͢۵Ŀհ״�ʹ�õ��������Ʒֱ�Ϊ �� ȫƷ��ѧ���� �ú��벻�ˣ���
��3����Ӧ��ʼǰ��������ȡSO2��װ����ͨ�봿����A�в������������������ǣ� ��
��4��װ��C�б��������� ��
��5��װ��D��Ŀ���� ȫƷ��ѧ���� �ú��벻�ˣ�
��6��д��ʵ�鲽����Թ��з�����Ӧ�Ļ�ѧ����ʽ �� ȫƷ��ѧ���� �ú��벻�ˣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ�������£�������һ������������Ӧ��NH3��NO�D��N2��H2O(δ��ƽ)���ڸ÷�Ӧ�У��������뱻��ԭ�ĵ�ԭ����֮��Ϊ(����)
A��2��3 B��3��2
C��4��5 D��5��6
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com