
���� ��1��þ�����ᷴӦ�����Ȼ�þ��������
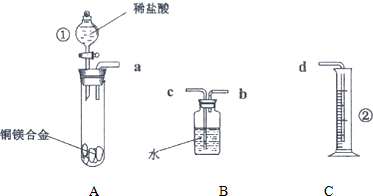
��2������װ��ͼ��֪�������ƣ�
��3����һ��������ͭþ�Ͻ�������ϡ���ᷴӦ���ⶨ��Ʒ��ͭ�������������Ǹ���þ�����ᷴӦ������������������þ����������ȷ��þ������������ʵ��������ˮ��������������������
��4��A�з�Ӧ��������������Կ��������ݣ��ݴ��жϷ�Ӧ�Ƿ���ϣ�
��5������ͬѧ����������Ϊ˼·�������Ĺ��弴Ϊͭ����������ͭ������������Ʒ��������Ϊͭ����������������ͬѧ������ˮ�������������Ϊ˼·���������������V����ܶȼ�����������������ٸ������������������þ������������Ʒ��������ȥþ��������Ϊͭ����������ͭ������������Ʒ��������Ϊͭ�������������ݴ˼��㣻
��6��a����ȡ��Ͳ��ˮ�����ʱ��δ��װ��������ָ������£�����õ����������ƫ��
b����ȡ��Ͳ��ˮ�����ʱ��������Ͳ������õ����������ƫ��
c��ʵ�鿪ʼǰ��û�м��װ�������ԣ�Ȼ��װ��ʵ����©���ģ����õ����������ƫС��
d��Bƿ�в����Ŀ����Ķ�ʵ����û��Ӱ�죻
e��δ��ȥ��Ʒͭþ�Ͻ���������Ĥ����г���������þ�����ᷴӦ������������ʹ�ü������þ������ƫС��
f������ˮ������������������ӷ�����HCl�����ʵ��û��Ӱ�죻
g��δ�Է�Ӧ��ʣ��IJ��������ϴ�ӣ�ʹ�ò�õ�ͭ������ƫ��
h��δ����B��Cװ�ü����ӵ����в�����ˮ�����õ����������ƫС��
�ݴ��жϣ�
��� �⣺��1��þ�����ᷴӦ�����Ȼ�þ����������Ӧ�Ļ�ѧ����ʽΪMg+2HCl=MgCl2+H2����
�ʴ�Ϊ��Mg+2HCl=MgCl2+H2����
��2������װ��ͼ��֪�����ٵ�����Ϊ��Һ©�����ڵ�����Ϊ��Ͳ��
�ʴ�Ϊ����Һ©������Ͳ��
��3����һ��������ͭþ�Ͻ�������ϡ���ᷴӦ���ⶨ��Ʒ��ͭ�������������Ǹ���þ�����ᷴӦ������������������þ����������ȷ��þ������������ʵ��������ˮ������������������������װ�õ�����˳��Ϊa��b��c��d��
�ʴ�Ϊ��b��c��
��4��A�з�Ӧ��������������Կ��������ݣ������жϷ�Ӧ��ϵ�����Ϊ��Һ��û���ݲ�����
�ʴ�Ϊ����Һ��û���ݲ�����
��5������ͬѧ����������Ϊ˼·�������Ĺ��弴Ϊͭ������������ͭ����������Ϊ$\frac{m{\;}_{2}}{m{\;}_{1}}$��100%������ͬѧ������ˮ�������������Ϊ˼·���������������V����ܶȼ��������������Ϊ��V��10-3g���ٸ���Mg+2HCl=MgCl2+H2������֪�μӷ�Ӧ��þ������Ϊ$\frac{24}{2}$����V��10-3g����Ʒ��ͭ������Ϊm1g-$\frac{24}{2}$����V��10-3g������ͭ����������Ϊ$\frac{m{\;}_{1}g-\frac{24}{2}����V��1{0}^{-3}}{m{\;}_{1}{g}_{\;}}$��100%=��$1-\frac{3��V}{250{m}_{1}}$����100%��
�ʴ�Ϊ��$\frac{m{\;}_{2}}{m{\;}_{1}}$�� ��$1-\frac{3��V}{250{m}_{1}}$����
��6��a����ȡ��Ͳ��ˮ�����ʱ��δ��װ��������ָ������£�����õ����������ƫ����ͭ����������С������ֵ��
b����ȡ��Ͳ��ˮ�����ʱ��������Ͳ������õ����������ƫ����ͭ����������С������ֵ��
c��ʵ�鿪ʼǰ��û�м��װ�������ԣ�Ȼ��װ��ʵ����©���ģ����õ����������ƫС������ͭ������������������ֵ��
d��Bƿ�в����Ŀ����Ķ�ʵ����û��Ӱ�죻
e��δ��ȥ��Ʒͭþ�Ͻ���������Ĥ����г���������þ�����ᷴӦ������������ʹ�ü������þ������ƫС������ͭ������������������ֵ��
f������ˮ������������������ӷ�����HCl�����ʵ��û��Ӱ�죻
g��δ�Է�Ӧ��ʣ��IJ��������ϴ�ӣ�ʹ�ò�õ�ͭ������ƫ����ͭ������������������ֵ��
h��δ����B��Cװ�ü����ӵ����в�����ˮ�����õ����������ƫС������ͭ������������������ֵ��
���Ե���ͭ������������������ֵ��ԭ������� cegh������ͭ����������С������ֵ��ԭ�������ab��
�ʴ�Ϊ��cegh��ab��
���� ������Ҫ����ʵ�������������Ʒ�ܳɳɷݵIJⶨ��ʵ����ƣ��Ѷ��еȣ�ע��ʵ��ԭ���ķ������������ʵ����������ͻ�ѧ��������֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2Na+2HCl�T2NaCl+H2�� | |
| B�� | Cu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$ CuSO4+SO2��+2H2O | |
| C�� | CuO+H2SO4�TCuSO4+H2O | |
| D�� | MnO2+4HCl��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$ MnCl2+Cl2��+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | һ������0.1mol/L | B�� | һ������0.1mol/L | ||
| C�� | һ��С��0.1mol/L | D�� | ��ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| ��Ӧʱ��/min | n��CO��/mol | n��H2O��/mol | n��CO2��/mol | n��H2��/mol |
| 0 | 1.2 | 0.60 | 0 | 0 |
| t1 | 0.80 | |||
| t2 | 0.20 |
| A�� | 0��t1 min�ڵ�ƽ����Ӧ����v��H2��=$\frac{0.2}{{t}_{1}}$mol•L-1•min-1 | |
| B�� | ƽ��ʱCO��ת����Ϊ66.67% | |
| C�� | t1ʱ�̸÷�Ӧ����ƽ��״̬ | |
| D�� | t2ʱ��CO��Ũ��Ϊ0.8 mol•L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ı�ȼ����Ϊ-890.3kJ•mol-1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��CH4��g��+2O2��g���TCO2��g��+2H2O��g����H=-890.3kJ•mol-1 | |
| B�� | 500�桢30MPa�£�0.5molN2��1.5molH2�����ܱ������г�ַ�Ӧ���ɰ���������19.3kJ�����Ȼ�ѧ����ʽΪ��N2��g��+3H2��g��?2NH3��g����H=-38.6kJ•mol-1 | |
| C�� | ��100�桢101kPa�����£�Һ̬ˮ��������Ϊ40.69kJ•mol-1����H2O��g��?H2O��l�� �ġ�H=+40.69 kJ•mol-1 | |
| D�� | �����кͷ�Ӧ����1molҺ̬H2Oʱ�����ų���������Ϊ�к��ȣ��ɱ�ʾΪ��H+��aq��+OH-��aq���TH2O��l����H=-57.3kJ•mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2Fe3++SO${\;}_{3}^{2-}$+H2O�T2Fe2++SO${\;}_{4}^{2-}$+2H+ | |
| B�� | 2H++SO${\;}_{3}^{2-}$�TH2O+SO2�� | |
| C�� | 2H++2NO${\;}_{3}^{-}$+3SO${\;}_{3}^{2-}$�T3SO${\;}_{4}^{2-}$+2NO��+H2O | |
| D�� | 2Fe3++3SO${\;}_{3}^{2-}$+3H2O�T2Fe��OH��3��+3SO2�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com