
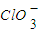 �����ֺ���Ԫ�ص����ӣ�����C1O-��
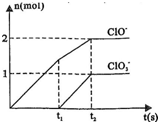
�����ֺ���Ԫ�ص����ӣ�����C1O-�� �������ӵ����ʵ�����n���뷴Ӧʱ�䣨t����������ͼ��ʾ��
�������ӵ����ʵ�����n���뷴Ӧʱ�䣨t����������ͼ��ʾ�� ______Cl-+______H2O��
______Cl-+______H2O��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2013?����ģ�⣩��֪Ca��OH��2��Cl2��Ӧ�������������¶��йأ���һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ�������ķ�Ӧ��Ϊ���ȷ�Ӧ�����������к���Cl-��ClO-��Cl
��2013?����ģ�⣩��֪Ca��OH��2��Cl2��Ӧ�������������¶��йأ���һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ�������ķ�Ӧ��Ϊ���ȷ�Ӧ�����������к���Cl-��ClO-��Cl| O | - 3 |
| O | - 3 |
| O | n- 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��֪Ca��OH��2��Cl2��Ӧ�������������¶��йأ���һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ�������ķ�Ӧ��Ϊ���ȷ�Ӧ�����������к���Cl-��ClO-��
��֪Ca��OH��2��Cl2��Ӧ�������������¶��йأ���һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ�������ķ�Ӧ��Ϊ���ȷ�Ӧ�����������к���Cl-��ClO-��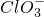 �����ֺ���Ԫ�ص����ӣ�����C1O-��
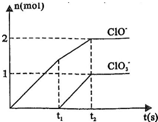
�����ֺ���Ԫ�ص����ӣ�����C1O-��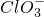 �������ӵ����ʵ�����n���뷴Ӧʱ�䣨t����������ͼ��ʾ��
�������ӵ����ʵ�����n���뷴Ӧʱ�䣨t����������ͼ��ʾ��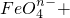 ______Cl-+______H2O��
______Cl-+______H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ģ�� ���ͣ������
| O | -3 |
| O | -3 |
| O | n-4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��д������������Ӧ�Ļ�ѧ����ʽ��
��SO2��CaCO3����Һ��Ӧ��____________________________________________________��
��SO2��Ca��OH��2����Һ��Ӧ��________________________________________________��
��2��˵������ʯ������Һ�����ó���ʯ��ˮϴ�ӷ�����ԭ��_______________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com