
 ���г����µ�������Һ��
���г����µ�������Һ��

 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��c��NH4+����ȵģ�NH4��2SO4��Һ����NH4��2Fe��SO4��2��Һ��NH4Cl��Һ�У�����Ũ�ȴ�С��ϵ�ǣ�c[��NH4��2Fe��SO4��2]��c[��NH4��2SO4]��c��NH4Cl�� |
| B����AgCl����Һ�е���KI��Һ��AgI�������ɣ�˵��AgCl���ܽ��С��AgI���ܽ�� |
| C��0.2 mol/L HCl��Һ������0.05 mol/L Ba��OH��2 ��Һ��Ϻ���Һ��pH=1 |
| D��0.2 mol/L��NaHCO3��Һ��c��H-��+c��Na+��=c��CO32-��+c��OH-��+c��HCO3-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��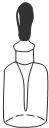 �õ�ƿ���ڱ���NaOH��Һ |
B�� ����ˮ������Ӧ |
C��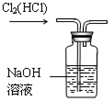 ��ȥCl2�е�HCl |
D��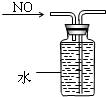 ��ˮ�������ռ�NO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Ba2+��OH-��NO3- |
| B��Ag+��HCO3-��NO3- |
| C��Mg2+��A13+��SO42- |
| D��Mg2+��SO42-��OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������Һ�У���ˮ�������������Ũ�Ⱦ�Ϊ1��10-11 mol?L-1 |
| B������Ũ��Ϊ����Ũ�ȵ�2�� |
| C�������е�c��CH3COO-���������е�c��SO42-����� |
| D���ֱ��������пƬ��������Һ����H2�������ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���õ�صĵ�ط�ӦʽΪ��2Li+FeS�TLi2S+Fe |
| B��Li-Al�Ͻ��ڵ������Ϊ�������� |
| C�������ĵ缫��ӦʽΪ��Al-3e-�TAl3+ |
| D��Li-Al/FeS����У����Ӿ����·����Li-Al�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com