
��֪�������ݣ�

ijѧ����ʵ������ȡ������������Ҫ�������£�
����30 mL�Ĵ��Թ�A�а������1��4��4�ı�������Ũ���ᡢ�Ҵ�������Ļ����Һ��
�ڰ���ͼ���Ӻ�װ��(װ������������)����С����ȵؼ���װ�л����Һ�Ĵ��Թ�5��10 min��

�۴��Թ�B�ռ���һ���������ֹͣ���ȣ������Թ�B��������Ȼ���ô��ֲ㣮
�ܷ�������������㣬ϴ�ӡ����
�������ĿҪ��ش��������⣺
(1)���Ƹû����Һ����Ҫ��������Ϊ��________��д����ȡ���������Ļ�ѧ����ʽ��________��
(2)����ʵ���б���̼������Һ��������________(����ĸ)��
A���к�������Ҵ�
B���к����Ტ���ղ����Ҵ�
C�����������ڱ���̼������Һ�е��ܽ�ȱ���ˮ�и�С�������ڷֲ�����
D�������������ɣ���������
(3)���������ҪС����ȼ��Ȳ���������Ҫ������________��
(4)ָ����������۲쵽������________������������������һ���ñ���ʳ��ˮ�ͱ����Ȼ�����Һϴ�ӣ���ͨ��ϴ�ӳ�ȥ________(������)���ʣ�Ϊ�˸���������������ѡ�õĸ����Ϊ________(����ĸ)��
A��P2O5
B����ˮNa2SO4
C����ʯ��
D��NaOH����
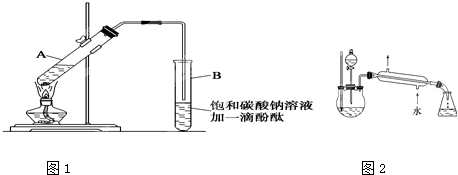
(5)ij��ѧ����С�����������ͼ��ʾ����ȡ����������װ��(ͼ�е�����̨�����С�����װ������ȥ)������ͼװ����ȣ���ͼװ�õ���Ҫ�ŵ��У�________��

|
�����𰸣�(1)��һ��30 mL���Թ���ע��4 mL�Ҵ����ٷֱ�����1 mL��Ũ���ᣬ�ӱ����Թܣ�����ȴ������ʱ���ټ���4 mL���Ტҡ�� ����CH3COOH��HOCH2CH3 ����(2)BC ����(3)��Ϊ��Ӧ���Ҵ�������ķе�ϵͣ����ô����ȣ���Ӧ������������������ʧԭ�ϣ��¶ȹ��߿��ܷ�����������Ӧ ����(4)��dz��ɫ̼������Һ���Ϸ�����ɫҺ�壬�ŵ���ζ����̼������Һ���ɫ��dz��̼���ơ��Ҵ���B ����(5)a���������¶ȼƣ������ڿ��Ʒ���װ���з�ӦҺ���¶ȣ�b�������˷�Һ©���������ڼ�ʱ���䷴Ӧ���Һ�������������������c��������ˮ����װ�ã��������ռ����� ����������(1)�����Ҵ���Ũ���ᡢ������Һʱ�����Լ������Թܵ�˳������Ϊ��CH3CH2OH ����CH3COOH��HOCH2CH3 ����(2)����̼������Һ��������Ҫ��3������ʹ�������������е�������Na2CO3��Ӧ����ȥ����ʹ������Ҵ��ܽ⣮��ʹ�����������ܽ�ȼ�С����������ļ����������ķֲ���ᴿ����ѡB��C� ����(3)�����и����ĸ����ʵ���Ҫ�������ʣ����ݸ����ʵķе����ݿ�֪������(117.9��)���Ҵ�(78.0��)�ķе㶼�Ƚϵͣ��������������ķе�(77.5��)�ȽϽӽ������ô����ȣ���Ӧ��������������(��������)һ��������������ԭ�ϵĴ�����ʧ����һ�����棬�¶�̫�ߣ����ܷ�����������Ӧ�� ����(4)�ڲ�����е���Ҫ�����ǣ��Թ�B�е�Һ��ֳ��������㣬�ϲ���״Һ����ɫ(�����ŵ�ˮ����ζ)���²�Һ��(dz)��ɫ�����²�Һ��ĺ�ɫ��dz������������Ǵֲ�Ʒ���������㣬���������ֲ�Ʒ���ᴿ��������Ϊ������ֲ�Ʒ�м���̼���Ʒ�ĩ(Ŀ���dz�ȥ�ֲ�Ʒ�е�����)���������м��뱥��ʳ��ˮ�뱥���Ȼ�����Һ�������á���Һ(Ŀ���dz�ȥ�ֲ�Ʒ�е��Ҵ�)���������м�����ˮ������(Ŀ���dz�ȥ�ֲ�Ʒ�е�ˮ)��������������������Һ�������һ���������ƿ�ڣ���������ȥ�ͷе���֣��ռ��¶���76��78��֮�����ּ��ô���������������ѡB� ����(5)�Ա�����ʵ��װ��ͼ������������Ʊ������еĸ����������ƣ����Կ������ߵ�����ͻ�����ŵ㣺���������¶ȼƣ����ڿ��Ʒ���װ���з�ӦҺ���¶ȣ����ٸ�����IJ������������˷�Һ©���������ڼ�ʱ���䷴Ӧ���Һ����������������IJ�������������ˮ����װ�ã��������ռ��������������� |


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij��ѧ��Ӧ�У��跴Ӧ���������ΪE1���������������ΪE2��
ij��ѧ��Ӧ�У��跴Ӧ���������ΪE1���������������ΪE2��
| �� | �۵�/�� | �ۻ�����/KJ?mol-1 | �ο��۸�/Ԫ?kg-1 |
| CaCL2?6H2O | 29��0 | 37��3 | 780��850 |
| Na2SO4?10H2O | 32��4 | 77��0 | 800��900 |
| Na2HPO4?12H2O | 36��1 | 100��1 | 1600��2000 |
| Na2S2O3?5H2O | 48��5 | 49��7 | 1400��1800 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� | �۵㣨�棩 | �е㣨�棩 | �ܶȣ�g/cm3�� |
| �Ҵ� | -117.0 | 78.0 | 0.79 |
| ���� | 16.6 | 117.9 | 1.05 |
| �������� | -83.6 | 77.5 | 0.90 |
| Ũ���ᣨ98%�� | - | 338.0 | 1.84 |
| Ũ���� |
| ���� |
| Ũ���� |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
���� | �۵㣨 �棩 | �е㣨 �棩 | �ܶ�(g��cm-3) |
�Ҵ� | -117.0 | 78.0 | 0.79 |
���� | 16.6 | 117.9 | 1.05 |
�������� | -83.6 | 77.5 | 0.90 |
Ũ���ᣨ98���� | ���� | 338.0 | 1.84 |
ѧ����ʵ������ȡ������������Ҫ�������£�
����30 mL�Ĵ��Թ�A�а������1��4��4�ı�������Ũ���ᡢ�Ҵ�������Ļ����Һ��
�ڰ���ͼ���Ӻ�װ�ã�װ�����������ã�����С����ȵؼ���װ�л����Һ�Ĵ��Թ�5��10 min��
�۴��Թ�B�ռ���һ���������ֹͣ���ȣ������Թ�B��������Ȼ���ô��ֲ㡣
�ܷ�������������㣬ϴ�ӡ����


ͼ1 ͼ2
�������ĿҪ������������⣺
��1�����Ƹû����Һ����Ҫ��������Ϊ��___________________________________________��
д����ȡ���������Ļ�ѧ����ʽ��________________________________________________��
��2������ʵ���б���̼������Һ�������ǣ�����ĸ����_______________��
A.�к�������Ҵ�
B.�к����Ტ���ղ����Ҵ�
C.���������ڱ���̼������Һ�е��ܽ�ȱ���ˮ�и�С�������ڷֲ�����
D.�����������ɣ���������
��3�����������ҪС����ȼ��Ȳ���������Ҫ�����ǣ�_______________________________��
��4��ָ����������۲쵽������_____________________________������������������һ���ñ���ʳ��ˮ�ͱ����Ȼ�����Һϴ�ӣ���ͨ��ϴ�ӳ�ȥ�������ƣ�_____________ �����ʣ�Ϊ�˸�������������ѡ�õĸ����Ϊ������ĸ����_____________��
A.P2O5 B.��ˮNa2SO4 C.��ʯ D.NaOH����
��5��ij��ѧ����С���������ͼ2��ʾ����ȡ����������װ�ã�ͼ�е�����̨�����С�����װ������ȥ������ͼ1װ����ȣ�ͼ2װ�õ���Ҫ�ŵ��У�_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
���� | �۵㣨 �棩 | �е㣨 �棩 | �ܶ�(g��cm-3) |
�Ҵ� | -117.0 | 78.0 | 0.79 |
���� | 16.6 | 117.9 | 1.05 |
�������� | -83.6 | 77.5 | 0.90 |
Ũ���ᣨ98���� | ���� | 338.0 | 1.84 |
ѧ����ʵ������ȡ������������Ҫ�������£�
����30 mL�Ĵ��Թ�A�а������1��4��4�ı�������Ũ���ᡢ�Ҵ�������Ļ����Һ��
�ڰ���ͼ���Ӻ�װ�ã�װ�����������ã�����С����ȵؼ���װ�л����Һ�Ĵ��Թ�5��10 min��
�۴��Թ�B�ռ���һ���������ֹͣ���ȣ������Թ�B��������Ȼ���ô��ֲ㡣
�ܷ�������������㣬ϴ�ӡ����


ͼ1 ͼ2
�������ĿҪ������������⣺
��1�����Ƹû����Һ����Ҫ��������Ϊ��___________________________________________��
д����ȡ���������Ļ�ѧ����ʽ��________________________________________________��
��2������ʵ���б���̼������Һ�������ǣ�����ĸ����_______________��
A.�к�������Ҵ�
B.�к����Ტ���ղ����Ҵ�
C.���������ڱ���̼������Һ�е��ܽ�ȱ���ˮ�и�С�������ڷֲ�����
D.�����������ɣ���������
��3�����������ҪС����ȼ��Ȳ���������Ҫ�����ǣ�_______________________________��
��4��ָ����������۲쵽������_____________________________������������������һ���ñ���ʳ��ˮ�ͱ����Ȼ�����Һϴ�ӣ���ͨ��ϴ�ӳ�ȥ�������ƣ�_____________ �����ʣ�Ϊ�˸�������������ѡ�õĸ����Ϊ������ĸ����_____________��
A.P2O5 B.��ˮNa2SO4 C.��ʯ D.NaOH����
��5��ij��ѧ����С���������ͼ2��ʾ����ȡ����������װ�ã�ͼ�е�����̨�����С�����װ������ȥ������ͼ1װ����ȣ�ͼ2װ�õ���Ҫ�ŵ��У�_____________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com