
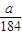

 ��
��
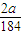
 ��mol����
��mol���� mol÷0.1L=
mol÷0.1L=
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��

 mol
mol mol×22.4L/mol=
mol×22.4L/mol= L���ʴ�Ϊ��
L���ʴ�Ϊ�� ��
��

 mol×22.4L/mol=
mol×22.4L/mol= L��
L��
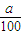
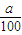 mol×22.4L/mol=
mol×22.4L/mol= L
L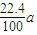 ��V
��V ��
��  ��V��
��V�� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣�����NaOH��NaHC03��������0��2 mol�����ܱ������м��ȵ�250�棬ʹ���ַ�Ӧ���ų����壬��ȴ���Ƶò�����������ΪW g��
(1)д�����ܷ�����Ӧ�Ļ�ѧ����ʽ��
(2)��������NaOHΪx mol(0<x<0��2)����xȡ��ֵͬʱ���ƶϲ������ʵ����࣬������W����ֵ(�����ú��С�x���Ĵ���ʽ��ʾ)��������ش�
_ Xֵ |
��������(��ѧʽ) |
Wֵ(g) |
X=0.1 |
|
|
0<x<0.1 |
|
|
0.1<x<O��2 |
|
|
(3)�����������У�����wֵ��xֵ�仯�����ߣ�

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com