
��08�꺣�Ͼ���(11��)��η�ֹ������ʴ�ǹ�ҵ���о����ص����ݡ�Ϊ�о�����ʴ��Ӱ�����أ�ijͬѧ��������̽��ʵ�飺
��� | ���� | ʵ������ |
1 | �����½���˿���ڸ��������һ���� | �������˿������Ȼ���� |
2 | �����½���˿���ڳ�ʪ������һСʱ | ��˿������Ȼ���� |
3 | �����½���˿���ڳ�ʪ�Ŀ�����һ���� | ��˿�����ѱ�ûҰ� |
4 | ����ʪ����˿���ڳ��µ���������һСʱ | ��˿�������ԻҰ� |
5 | ����ʪ����˿���ڸ��ڳ��µ���������һСʱ | ��˿�����ѱ�ûҰ� |
6 | �������Ȼ�����Һ����˿���ڸ��ڳ��µ���������һСʱ | ��˿����Ұ��̶ȱ�ʵ��5���� |
�ش��������⣺
(1)����ʵ���з����˵绯ѧ��ʴ����(��ʵ�����) __________; �ڵ绯ѧ��ʴ�У�������Ӧ��________________; ������Ӧ��_______________________��
(2)�ɸ�ʵ���֪������Ӱ������ʴ���ʵ�������_________________________��
(3)Ϊ��ֹ������ʴ����ҵ���ձ���õķ�����______________(�����ַ���)
����(1)3��4��5��6 ������Fe-2e��Fe2+ ������O2+2H2O+4e��4OH-
(2) �¶ȡ�ʪ�ȡ�����ʴ��ڡ�O2��Ũ��
(3) ����ӱ������磺Ϳ�ᡢ�´ɡ���ơ�����ۻ�����֬�ȡ�
�ڸı�������ڲ��ṹ��ұ������֡�
��������ҵ��������ʴ��Ҫ�ǵ绯ѧ��ʴ�������绯ѧ��ʴ�������ǣ����Բ�ͬ���������ϣ��������Һ���γɱպϻ�·��Ӱ����ʴ�ٶȵ������У��������¶ȡ�ʪ�ȡ�����ʴ��ڡ�O2��Ũ�ȵȡ�ʵ������Ϊ������Ϊ������ʴ��
������Fe-2e��Fe2+ ������O2+2H2O+4e��4OH-
��������������ʴ�Ĵ�ʩ
����ӱ������磺Ϳ�ᡢ�´ɡ���ơ�����ۻ�����֬�ȡ�
�ڸı�������ڲ��ṹ��ұ������֡�
���ڸ����ı�����Ƕ�������õĽ�����п��ʹ���ý�������������������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)�÷�Ӧ��____________________��Ӧ(����ȡ������ȡ�);
(2)����Ӧ�ﵽƽ��ʱ�������¶ȣ�A��ת����______(�������С�������䡱)��
ԭ����____________________________________________��
(3)��Ӧ��ϵ�м�������Է�Ӧ���Ƿ���Ӱ��?_______________��ԭ����_________��
(4)�ڷ�Ӧ��ϵ�м����������Ӧ��������E1��E2�ı仯�ǣ�E1_________��E2________(�������С���������䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08�꺣�Ͼ���(8��)��ӦA(g)+B(g)�� ![]() C(g) +D(g)�����е������仯��ͼ��ʾ���ش��������⡣
C(g) +D(g)�����е������仯��ͼ��ʾ���ش��������⡣
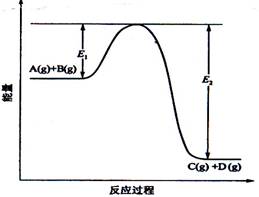
(1)�÷�Ӧ��____________________��Ӧ(����ȡ������ȡ�);
(2)����Ӧ�ﵽƽ��ʱ�������¶ȣ�A��ת����______(�������С�������䡱)��
ԭ����____________________________________________��
(3)��Ӧ��ϵ�м�������Է�Ӧ���Ƿ���Ӱ��?_______________��ԭ����_________��
(4)�ڷ�Ӧ��ϵ�м����������Ӧ��������E1��E2�ı仯�ǣ�E1_________��E2________(�������С���������䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(08�꺣�Ͼ�)(10��)A��B��C��D��E��Ϊ�л����������֮��Ĺ�ϵ��ͼ��ʾ(��ʾ��RCH=CHR'�����Ը��������Һ�з�Ӧ����RCOOH��R'COOH������R��R'Ϊ���)��

�ش��������⣺
(1)ֱ��������A����Է�������С��90��A������̼����Ԫ�ص�����������Ϊ0.814������Ϊ��Ԫ�أ���A�ķ���ʽΪ_____________��
(2)��֪B��NaHCO3��Һ��ȫ��Ӧ�������ʵ���֮��Ϊ1��2������Ũ����Ĵ��£�B��������C2H5OH������Ӧ�Ļ�ѧ����ʽ��___ ��Ӧ����Ϊ_____��
(3)A��������������÷ų���������ʹ������Ȼ�̼��Һ��ɫ����A�Ľṹ��ʽ��__________________
(4)D��ͬ���칹���У�����NaHCO3��Һ��Ӧ�ų�CO2����__________�֣�����Ӧ�Ľṹ��ʽ��__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08�꺣�Ͼ���п���ϡ�����ᷴӦ��������п������狀�ˮ��������1 mol����пʱ������ԭ����������ʵ���Ϊ�� ��
A. 2mol B. 1 mol C. 0.5mol D. 0.25mol
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com