
����Ŀ����1����5.1gþ���Ͻ�ķ�ĩ��������������У��õ�5.6 LH2����״���£���
�ٺϽ���þ�����ʵ���_________��
��д���úϽ���������NaOH��Һ�Ļ�ѧ����ʽ_________________��ͬʱ����H2���������״���£�Ϊ____________ ��
��2����ͬ�����£�ijCl2��O2�������100 mLǡ����150 mL H2��������HCl��H2O������������Cl2��O2�������Ϊ__________����������ƽ����Է�������Ϊ____________��
��3��������ͬ�ݻ����ܱ�����X��Y����25 ���£�X�г���a g A���壬Y�г���a g CH4���壬X��Y�ڵ�ѹǿ֮����4��11����A��Ħ������Ϊ____________��
��4����ͬ�¡�ͬѹ�£�ʵ����CO��N2��O2��������Ļ��������ܶ���H2��14.5��������O2����������Ϊ____________ (����������1λС��) ��������CO��N2�����ʵ���֮��Ϊ1��1��������������Ԫ�ص���������Ϊ_______________ (����������1λС��)��
���𰸡�0.1 mol 2Al+2NaOH+2H2O=2NaAlO2+3H2�� 3.36 L 1��1 27.25 44 g��mol��1 27.6% 48.3%
��������
��1���ٸ���þ����������������������з�������㣻
��ֻ�������������Ʒ�Ӧ�����м��㣻
��2���з�������㣬��������ƽ����Է����������ڻ����������������Ի������������ʵ���������ֵ��
��3������PV=nRT���м��㣻
��4����ͬ�¡�ͬѹ�£��ܶȱȵ�����Է�������֮�Ƚ��м��㣻�����������Ԫ�ص���������Ϊ��Ԫ�ص����������������������
����þ���������ʵ����ֱ�Ϊ x mol�� y mol���з����飺
��24x +27y =5.1 ��x + 1.5y =5.6/22.4=0.25���٢�������x=0.1mol��y=0.1mol
�𰸣�0.1mol
��д���úϽ���������NaOH��Һ�Ļ�ѧ����ʽ2Al+2NaOH+2H2O=2NaAlO2+3H2����
��Ϊ2Al��3H2��ǰ���Ѿ����Al�����ʵ���Ϊ0.1mol����V��H2��=0.1mol��3/2��22.4L/mol=3.36L��
�𰸣�2Al+2NaOH+2H2O=2NaAlO2+3H2�� 3.36L��
��2��������Ϊxml������Ϊyml
Cl2 + H2![]() 2HCl��2H2 + O2
2HCl��2H2 + O2![]() 2H2O
2H2O
xml xml 2yml yml
��x+y=100 �� x+2y=150���٢��������������ɵ�x=y=50ml��
����������������1Ħ�������������ƽ��Ħ������Ϊ��![]() =51.5g/mol�����������ƽ����Է�������Ϊ51.5��
=51.5g/mol�����������ƽ����Է�������Ϊ51.5��
�𰸣�1��1��51.5
��3����1������PV=nRT����T��V��ͬʱ��ѹǿ֮�ȵ������ʵ���֮�ȣ���4��11=![]() ��
��![]() ����ã�M=44g/mol��
����ã�M=44g/mol��
�𰸣�44g/mol��
��4�����������ܶ���H2��14.5��,ƽ����Է�������=14.5��2=29,����CO,N2��Է�����������28,���Կ�����CO,N2�����ʵ���Ϊx,O2���ʵ���Ϊy,��28x+32y=29��(x+y),���x:y=3:1,����O2����������=32/(29��4)=27.6%
������CO��N2�����ʵ���֮��Ϊ1:1,����COΪ1mol,����Ϊ1mol������Ϊ��1+1����1/3=2/3mol,������������Ԫ�ص���������= ��100%=48.3%��
��100%=48.3%��
�𰸣�27.6% 48.3%


 �����ܾ�ϵ�д�
�����ܾ�ϵ�д� ���ƿ�����ϵ�д�
���ƿ�����ϵ�д� ���¿쳵����������ϵ�д�
���¿쳵����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2 L�ܱ������н��з�Ӧ��pZ(g)��qQ(g) ![]() mX(g)��nY(g)��ʽ��m��n��p��qΪ��ѧ����������0��3 min�ڣ������ʵ����ʵ����ı仯���±���ʾ��
mX(g)��nY(g)��ʽ��m��n��p��qΪ��ѧ����������0��3 min�ڣ������ʵ����ʵ����ı仯���±���ʾ��
���� | X | Y | Z | Q |
��ʼ/mol | 0.7 | 1 | ||
2 minĩ/mol | 0.8 | 2.7 | 0.8 | 2.7 |
3 minĩ/mol | 0.8 |
��֪��2 min��v(Q)��0.075 mol��L��1��min��1��v(Z)��v(Y)��1��2��
��ش��������⣺
(1)2 min��X�ķ�Ӧ����v(X)��__________��
(2)��ʼʱn(Y)��__________��
(3) 3 minĩ�Ƿ�ﵽƽ��_______(���ǻ��)��
(4)���ڸ÷�Ӧ������������Ӧ���ʵĴ�ʩ��________(����ţ���ͬ)��
A����С������� B�����߲���Q
C��ͨ�����He�� D�������¶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ����д���пհ�
����ͼ��ʾ��a��b��Ϊ�������ɿ��������ͨ����Һ��ļ��룬ʵ��ǰ�������ѹرգ������Ĺ̶�װ����ʡ�ԣ�����ѡ�������˵��Լ���������ͼ����һ������CO2��CO��������е�CO2��CO���롣�ɹ�ѡ����Լ��У�Ũ���ᡢϡ���ᡢŨ���ᡢϡ���ᡢ����������Һ����ɫʯ����Һ��

��1����װ�õ���ƿ��ʢ�ŵ��Լ���_____________����Һ©����ʢ�ŵ��Լ���__________��
��2����װ�õ�ƿ��ʢ�ŵ��Լ���_______________��
��3������aʱ�����������������________��Ҫ�õ���һ������ʱ����ȷ�IJ�����_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪Fe3O4�ɱ�ʾ��FeO��Fe2O3,ˮ�ȷ��Ʊ�Fe3O4���������ܷ�ӦΪ:3Fe2++2S2O32��![]() +O2+4OH-
+O2+4OH-![]() Fe3O4+S4O62-
Fe3O4+S4O62-![]() +2H2O������˵����ȷ����(����)
+2H2O������˵����ȷ����(����)
A. O2��S2O32����������,Fe2+�ǻ�ԭ��
B. ����2 mol Fe2+������,��Fe2+��ԭ��O2Ϊ0.5 mol
C. ÿ����1 mol Fe3O4 ,ת�Ƶ��ӵ����ʵ���Ϊ2 mol
D. �μӷ�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ1��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ��þ���Ͻ�Ͷ�뵽1 mol/L���У����Ͻ���ȫ�ܽ��������Һ�����1 mol/L NaOH��Һ�����ɳ��������ʵ��������NaOH��Һ������仯�Ĺ�ϵ����ͼ��ʾ������˵���д�����ǣ� ��

A. ��������Ϊ80mlB. a��ȡֵ��ΧΪ0�� a ��50
C. n(Mg2+)��0.025molD. ��aֵΪ30ʱ��bֵΪ0.01
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ���ǣ� ��
A. ��ϩʹ��ˮ�����Ը��������ɫ�����ڼӳɷ�Ӧ
B. ������ˮ�ܼ��𱽡��Ҵ�������������Һ
C. ���ظ������Һ����˾���Ƿ�Ƽ��������ķ�Ӧ�����Ҵ���������Ӧ
D. ʯ�͵ķ���ú�ĸ����������仯�����ѻ����ѽ��ǻ�ѧ�仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ƶõ�̼������Ʒ��������������NaCl�������ⶨ��Ʒ��Na2CO3������������ij̽����ѧϰС��ֱ����������ʵ�鷽������ش������й����⣺
����һ����һ����������Ʒ�ܽ����������CaCl2��Һ�������ó���____________(���������)��ϴ�ӡ���ɡ����������㡣ϴ�ӳ����ľ��������_____________________________��
����������һ��������Ʒ���������ᷴӦ������ͼ��ʾװ�òⶨ����CO2�������Ϊ��ȷ���ⶨ�����ȷ�ԣ�B�е���Һ��ò���_________________����ͼװ����ʵ������a������_________________��

��������������ͼ��ʾװ�����ⶨ������Ʒ��̼���Ƶ���������������̨�����е���ͼ�о�����ȥ����ʵ�鲽�����£�
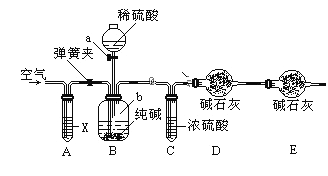
�ٰ�ͼ����װ�ã�����������ԣ�
��ȷ�Ƶ�ʢ�м�ʯ�ҵĸ����D������Ϊ33.4g��
��ȷ�Ƶ�6g������Ʒ��������b�У�
�ܴ�Һ©��a����������������ϡ���ᣬ�����ٲ�������Ϊֹ��
�ݴ��ɼУ����Թ�A�л���������������ӣ�Ȼ��Ƶø����D��������Ϊ35.6g��
��1�����ܢ�������ʵ�����̫�죬��ᵼ�²ⶨ���__________������ƫ��������ƫС������
��2��װ��A���Լ�XӦѡ��________________________��
��3��Eװ�õ�������_______________________________��
��4������ʵ���в�õ��й����ݣ����㴿����ƷNa2CO3����������Ϊ___________���������С�����һλ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ������ʵ��װ�ûش����⡣
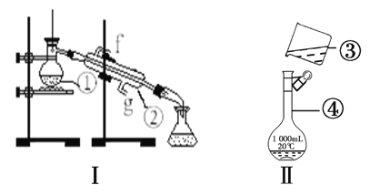
��1��д���������������ƣ�______________��___________________��
��2�������١����У�ʹ��ʱ�������Ƿ�©ˮ����____________________��������ţ�
��3����װ��I������ˮ����ȱ�ٵ�������_________����ȴˮ��_______�ڽ���
��4��a����������250mL0.2molL-1NaCl��Һ������װ��II��ijͬѧ���ƴ���Һʱת�Ʋ�����ʾ��ͼ��ͼ������������ֱ���_______________��_______________��
b�����ƹ����У����������ʹ���ƽ��ƫ�ߵ���________________������ţ���
�ٶ���ʱ���ӿ̶��߹۲�Һ�棻������ƿʹ��ʱδ����۶��ݺ���ҡ�ȡ����ã�����Һ����ڿ̶��ߣ��ټ�����ˮ�����̶��ߣ�����Һʱδϴ���ձ��Ͳ�����
c����ʵ���м�����ˮʱ���������˿̶ȣ�Ӧ��δ�����______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������װ�ÿ�������NH3��SO2��������Ʊ���

��ش��������⣺
��1������Ca(OH)2�����NH4Cl������ȡ����NH3����ѡ�õ�װ����_____������ţ�����NH3�Ļ�ѧ����ʽΪ__________________________________������NH3������____________���Լ����ƣ������鼯��ƿ���Ƿ��ռ��������ķ�����____________________________________��
��2������ͭ��Ũ���ᷴӦ��ȡSO2��Ӧѡ��װ��________������ţ�������SO2�Ļ�ѧ����ʽΪ_____________________________________________________��������ţ�
������Һ��ֱ�����ڼ���SO2������ڵ���__________��
A������KMnO4��Һ B��NaOH��Һ C��Na2CO3��Һ D��Ʒ����Һ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com