
£Ø14·Ö£©ĢžĶعż³ōŃõ»Æ²¢¾ŠæŗĶĖ®“¦ĄķµĆµ½Č©»ņĶŖ”£
ĄżČē£ŗ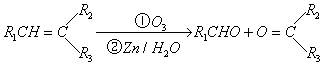
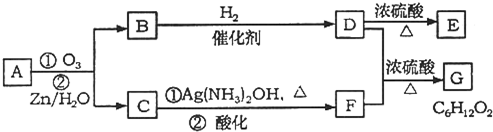
ÉĻŹö·“Ó¦æÉÓĆĄ“ĶʶĻĻ©ĢžµÄ½į¹¹”£Ņ»ÖÖĮ“דµ„Ļ©ĢžAĶعż³ōŃõ»Æ²¢¾ŠæŗĶĖ®“¦ĄķµĆµ½BŗĶC”£»ÆŗĻĪļBµÄĻą¶Ō·Ö×ÓÖŹĮæŹĒ58£¬ŗ¬Ģ¼62.1%£¬ŗ¬Ēā10.3%£¬BĪŽŅų¾µ·“Ó¦£¬“߻ƼÓĒāÉś³ÉD£¬DŌŚÅØĮņĖį“ęŌŚĻĀ¼ÓČČ£¬æɵƵ½ÄÜŹ¹äåĖ®ĶŹÉ«µÄĪļÖŹE”£·“Ó¦Ķ¼Ź¾ČēĻĀ£ŗ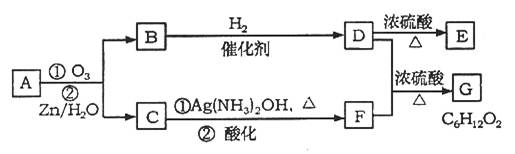
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£© µÄ·“Ó¦ĄąŠĶĪŖ_____________£»CÖŠŗ¬ÓŠ¹ŁÄÜĶŵÄĆū³ĘĪŖ___________£»BµÄ½į¹¹¼ņŹ½ĪŖ _________________”£
µÄ·“Ó¦ĄąŠĶĪŖ_____________£»CÖŠŗ¬ÓŠ¹ŁÄÜĶŵÄĆū³ĘĪŖ___________£»BµÄ½į¹¹¼ņŹ½ĪŖ _________________”£
£Ø2£© µÄ»Æѧ·½³ĢŹ½ŹĒ__________________________________”£
µÄ»Æѧ·½³ĢŹ½ŹĒ__________________________________”£
£Ø3£©AµÄ½į¹¹¼ņŹ½ĪŖ__________________________________________”£
£Ø4£©»ÆŗĻĪļAµÄijÖÖĶ¬·ÖŅģ¹¹ĢåĶعż³ōŃõ»Æ²¢¾ŠæŗĶĖ®“¦ĄķÖ»µĆµ½Ņ»ÖÖ²śĪļ£¬Š“³öĖłÓŠ·ūŗĻøĆĢõ¼žµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½__________________”¢_____________”£
 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
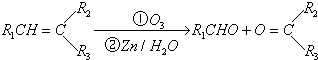
| ÅØĮņĖį |
| ”÷ |
| ÅØĮņĖį |
| ”÷ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĄżČē£ŗ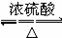
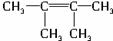
ÉĻŹö·“Ó¦æÉÓĆĄ“ĶʶĻĻ©ĢžµÄ½į¹¹”£Ņ»ÖÖĮ“דµ„Ļ©ĢžAĶعż³ōŃõ»Æ²¢¾ŠæŗĶĖ®“¦ĄķµĆµ½BŗĶC”£»ÆŗĻĪļBŗ¬Ģ¼62.1%£¬ŗ¬Ēā10.3%£¬BĪŽŅų¾µ·“Ó¦£¬“߻ƼÓĒāÉś³ÉD£¬DŌŚÅØĮņĖį“ęŌŚĻĀ¼ÓČČ£¬æɵƵ½ÄÜŹ¹äåĖ®ĶŹÉ«µÄĪļÖŹE”£·“Ó¦Ķ¼Ź¾ČēĻĀ£ŗ?
»Ų“šĻĀĮŠĪŹĢā£ŗ?
£Ø1£©D”śEµÄ·“Ó¦ĄąŠĶĪŖ£»CÖŠŗ¬ÓŠ¹ŁÄÜĶŵÄĆū³ĘĪŖ£»BµÄĻą¶Ō·Ö×ÓÖŹĮæŹĒ_____________”£
£Ø2£©D+F”śGµÄ»Æѧ·½³ĢŹ½ŹĒ£ŗ”””””””””””””””””””””£
£Ø3£©AµÄ½į¹¹¼ņŹ½ĪŖ____________________________”£
£Ø4£©»ÆŗĻĪļAµÄijÖÖĶ¬·ÖŅģ¹¹ĢåĶعż³ōŃõ»Æ²¢¾ŠæŗĶĖ®“¦ĄķÖ»µĆµ½Ņ»ÖÖ²śĪļ£¬Š“³öĖłÓŠ·ūŗĻøĆĢõ¼žµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½”£_________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011ÄźÕć½Ź”ø߶ž½×¶Ī12ŌĀ¼ģ²ā»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£©Ļ©ĢžĶعż³ōŃõ»Æ²¢¾ŠæŗĶĖ®“¦ĄķµĆµ½Č©»ņĶŖ”£ĄżČē£ŗ
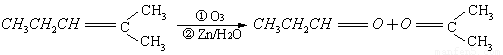
ÉĻŹö·“Ó¦æÉÓĆĄ“ĶʶĻĻ©ĢžµÄ½į¹¹”£Ņ»ÖÖĮ“דµ„Ļ©ĢžAĶعż³ōŃõ»Æ²¢¾ŠæŗĶĖ®“¦ĄķµĆµ½BŗĶC”£»ÆŗĻĪļBŗ¬Ģ¼69.8%£¬ŗ¬Ēā11.6%£¬BĪŽŅų¾µ·“Ó¦£¬“߻ƼÓĒāÉś³ÉD”£DŌŚÅØĮņĖį“ęŌŚĻĀ¼ÓČČ£¬æɵƵ½ÄÜŹ¹äåĖ®ĶŹÉ«ĒŅÖ»ÓŠŅ»ÖÖ½į¹¹µÄĪļÖŹE”£·“Ó¦Ķ¼Ź¾ČēĻĀ£ŗ
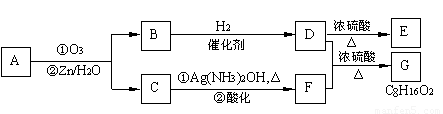
£Ø1£©AµÄ½į¹¹¼ņŹ½ĪŖ ”£
£Ø2£©D ”ś EµÄ·“Ó¦ĄąŠĶĪŖ £»BÖŠŗ¬ÓŠ¹ŁÄÜĶŵÄĆū³Ę ”£GĪļÖŹÖŠÓŠĪŽŹÖŠŌĢ¼Ō×Ó£æ ”£DĪļÖŹµÄŗĖ“Ź²ÕńĘ×Ķ¼ÖŠÓŠ¼øøö·å£æ ”£
£Ø3£©Š“³ö»Æѧ·½³ĢŹ½£ŗ
C”śF ”£
 ӣ
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗĢģ½ņøßæ¼ÕęĢā ĢāŠĶ£ŗĶʶĻĢā

 £¬±ūĶŖµÄČ¼ÉÕČČĪŖ1789
£¬±ūĶŖµÄČ¼ÉÕČČĪŖ1789 £¬ŹŌŠ“³ö±ūČ©Č¼ÉÕµÄČČ»Æѧ·½³ĢŹ½_____________ ”£
£¬ŹŌŠ“³ö±ūČ©Č¼ÉÕµÄČČ»Æѧ·½³ĢŹ½_____________ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com