



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®50£®8 g | B£®66£®4 g | C£®44£®8g | D£®39£®2g |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®H2O2”¢Na2O2¶¼ŹōÓŚ¹żŃõ»ÆĪļ£¬¶¼“ęŌŚ¹²¼Ū¼ü |
| B£®Ė«ŃõĖ®ŹĒĀĢÉ«Ńõ»Æ¼Į£¬æÉ×÷Ņ½ĮĘĻū¶¾¼Į |
| C£®H2O2ŌŚ¹żŃõ»ÆĒāĆøµÄ“ß»ÆĻĀ£¬ĪĀ¶ČŌ½øߣ¬·Ö½āĖŁĀŹŌ½æģ |
| D£®H2O2×öĘÆ°×¼ĮŹĒĄūÓĆĘäŃõ»ÆŠŌ£¬ĘÆ°×ŌĄķÓėHClOĄąĖĘ”¢ÓėSO2²»Ķ¬ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
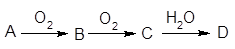
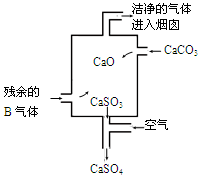
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗ¼ĘĖćĢā
| | NaCl | NaHCO3 | NH4Cl |
| 10”ę | 35.8 | 8.15 | 33.0 |
| 45”ę | 37.0 | 14.0 | 50.0 |
 £Ø4£©ĒėĢÖĀŪbØMaµÄȔֵ·¶Ī§¼°ÓėÖ®¶ŌÓ¦µÄČÜŅŗµÄČÜÖŹ¼°ĘäĪļÖŹµÄĮ棬½«½į¹ūĢīÓŚĻĀ±ķÖŠ£ŗ
£Ø4£©ĒėĢÖĀŪbØMaµÄȔֵ·¶Ī§¼°ÓėÖ®¶ŌÓ¦µÄČÜŅŗµÄČÜÖŹ¼°ĘäĪļÖŹµÄĮ棬½«½į¹ūĢīÓŚĻĀ±ķÖŠ£ŗ| bØMaµÄȔֵ·¶Ī§ | ČÜÖŹ | ČÜÖŹĪļÖŹµÄĮæ |
| | | |
 | ”Ŗ”Ŗ | ”Ŗ”Ŗ |
| | | |
| | | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®NaN3ÖŠÖ»ŗ¬ÓŠĄė×Ó¼ü |
| B£®1molNaN3ĶźČ«·Ö½ā×ī¶ąæÉŅŌ²śÉś33.6LN2 |
| C£®NaN3æģĖŁ·Ö½āŹ±£¬NaN3¼ČŹĒŃõ»Æ¼Į£¬ÓÖŹĒ»¹Ō¼Į |
| D£®³£ĪĀĻĀ£¬NaN3»ÆѧŠŌÖŹĪČ¶Ø£¬°ü×°ŗŠÉĻĪŽŠčĢŲ±šĖµĆ÷×¢ŅāŹĀĻī |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
A£®£Ø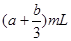 | B£® | C£® | D£®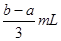 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com