
¹¤ŅµĮ¶ĀĮŹĒÓĆĀĮĶĮæó£ØÖ÷ŅŖ³É·ÖŹĒAl2O3£¬»¹ÓŠÉŁĮæµÄFe2O3”¢SiO2£©ĢįČ”Ņ±Į¶ĀĮµÄÖ÷ŅŖŌĮĻŃõ»ÆĀĮ£¬Č»ŗó½ųŠŠµē½ā”£¹¤ŅÕĮ÷³ĢČēĻĀĶ¼£ŗ
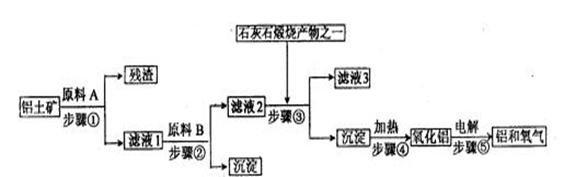
£Ø1£©ĀĖŅŗ1ÖŠŅŖ¼ÓČėÉŌ¹żĮæµÄŌĮĻB£¬ŌĮĻBµÄ»ÆѧŹ½ŹĒ”””””””” ”£Š“³ö²½Öč¢ŚÓŠ¹Ų·“Ó¦µÄĄė×Ó·½ ”¢ ”£
£Ø2£©Čē¹ūŹ”Č„²½Öč¢Ł£¬¼“ČܽāĀĮĶĮæó“ÓÖ±½Ó¼ÓČėŌĮĻBæŖŹ¼£¬Ōņ×īÖÕ»į¶ŌŃõ»ÆĀĮµÄÉś³ÉÓŠŹ²Ć“Ó°Ļģ£æ”””” ”£
£Ø3£©²½Öč¢ŻŹĒŌŚĀĮµē½ā²ŪÖŠ½ųŠŠ£¬µē½ā²ŪµÄĮ½¼«²ÄĮĻ¾łÓĆŹÆÄ«£¬ĘäÖŠ”” ¼«µÄ²ÄĮĻĖę·“Ó¦µÄ½ųŠŠŠčŅŖ²»¶Ļ²¹³ä£¬ĪŖŹ²Ć“£æ””””””””””
£Ø4£©ĀĮ·ŪÓėČżŃõ»Æ¶žĢś·ŪÄ©ŌŚŅżČ¼¼Į×÷ÓĆĻĀ³£ÓĆĄ“ŗø½ÓøÖ¹ģ£¬Ö÷ŅŖŹĒĄūÓĆĮĖøĆ·“Ó¦”””””” ”””””” £¬¶ųĒŅøĆ·“Ó¦ŗø½ÓĖŁ¶Čæģ”¢Éč±ø¼ņŅ×£¬ŹŹÓŚŅ°Ķā×÷Ņµ”£
£Ø1£©NaOH £Ø1·Ö£©
Al3++4OH£===AlO2£+2H2O £Ø1·Ö£© Fe3++3OH£===Fe(OH)3”ż£Ø1·Ö£©
£Ø2£©ÓĆNaOHČÜŅŗÖ±½ÓČܽā½«µ¼ÖĀSiO2Éś³ÉNaSiO3£¬ĶØCO2ŗóÉś³É¹čĖįŗĶĒāŃõ»ÆĀĮ³Įµķ£¬“Ó¶ų¼ÓČČŗóÉś³ÉŃõ»ÆĀĮÖŠŗ¬ÓŠŌÓÖŹ¶žŃõ»Æ¹č”££Ø2·Ö£©
£Ø3£©Ńō£Ø1·Ö£© Ńō¼«Éś³ÉµÄŃõĘų»įÓėŹÆÄ«·“Ӧɜ³ÉCO2¶ųĻūŗÄ£¬Ņņ“ĖŠčŅŖ²»¶Ļ²¹³ä£Ø2·Ö£©
£Ø4£©·Å³ö“óĮæµÄČČ£Ø1·Ö£©


 æŚĖćÄÜŹÖĻµĮŠ“š°ø
æŚĖćÄÜŹÖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ½Ī÷Ź”ÄĻ²żŅ»ÖŠ”¢ÄĻ²żŹ®ÖŠ2011½ģøßČżµŚŅ»“ĪĮŖæ¼»ÆѧŹŌĢā ĢāŠĶ£ŗ022
¹¤ŅµĮ¶ĀĮŹĒÓĆĀĮĶĮæó(Ö÷ŅŖ³É·ÖŹĒAl2O3£¬»¹ÓŠÉŁĮæµÄFe2O3”¢SiO2)ĢįČ”Ņ±Į¶ĀĮµÄÖ÷ŅŖŌĮĻŃõ»ÆĀĮ£¬Č»ŗó½ųŠŠµē½ā£®¹¤ŅÕĮ÷³ĢČēĻĀĶ¼£ŗ

(1)ĀĖŅŗ1ÖŠŅŖ¼ÓČėÉŌ¹żĮæµÄŌĮĻB£¬ŌĮĻBµÄ»ÆѧŹ½ŹĒ________£®Š“³ö²½Öč¢ŚÓŠ¹Ų·“Ó¦µÄĄė×Ó·½________”¢________£®
(2)Čē¹ūŹ”Č„²½Öč¢Ł£¬¼“ČܽāĀĮĶĮæó“ÓÖ±½Ó¼ÓČėŌĮĻBæŖŹ¼£¬Ōņ×īÖÕ»į¶ŌŃõ»ÆĀĮµÄÉś³ÉÓŠŹ²Ć“Ó°Ļģ£æ________£®
(3)²½Öč¢ŻŹĒŌŚĀĮµē½ā²ŪÖŠ½ųŠŠ£¬µē½ā²ŪµÄĮ½¼«²ÄĮĻ¾łÓĆŹÆÄ«£¬ĘäÖŠ________¼«µÄ²ÄĮĻĖę·“Ó¦µÄ½ųŠŠŠčŅŖ²»¶Ļ²¹³ä£¬ĪŖŹ²Ć“£æ________
(4)ĀĮ·ŪÓėČżŃõ»Æ¶žĢś·ŪÄ©ŌŚŅżČ¼¼Į×÷ÓĆĻĀ³£ÓĆĄ“ŗø½ÓøÖ¹ģ£¬Ö÷ŅŖŹĒĄūÓĆĮĖøĆ·“Ó¦________£¬¶ųĒŅøĆ·“Ó¦ŗø½ÓĖŁ¶Čæģ”¢Éč±ø¼ņŅ×£¬ŹŹÓŚŅ°Ķā×÷Ņµ£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

(1)ĀĖŅŗ1ÖŠŅŖ¼ÓČėÉŌ¹żĮæµÄŌĮĻB£¬ŌĮĻBµÄ»ÆѧŹ½ŹĒ__________________________”£
(2)Čē¹ūŹ”Č„²½Öč¢Ł£¬¼“ČܽāĀĮĶĮæó“ÓÖ±½Ó¼ÓČėŌĮĻBæŖŹ¼£¬Ōņ×īÖÕ»į¶ŌŃõ»ÆĀĮµÄÉś³ÉÓŠŹ²Ć“Ó°Ļģ£æ_______________________________________”£
(3)²½Öč¢ŻŹĒŌŚĀĮµē½ā²ŪÖŠ½ųŠŠ£¬µē½ā²ŪµÄĮ½¼«²ÄĮĻ¾łÓĆŹÆÄ«£¬ĘäÖŠ_____________¼«µÄ²ÄĮĻĖę·“Ó¦µÄ½ųŠŠŠčŅŖ²»¶Ļ²¹³ä£¬ĪŖŹ²Ć“£æ_______________________________________”£
(4)ĀĮ·ŪÓėČżŃõ»Æ¶žĢś·ŪÄ©ŌŚŅżČ¼¼Į×÷ÓĆĻĀ³£ÓĆĄ“ŗø½ÓøÖ¹ģ£¬Ö÷ŅŖŹĒĄūÓĆĮĖøĆ·“Ó¦_____________£¬¶ųĒŅøĆ·“Ó¦ŗø½ÓĖŁ¶Čæģ”¢Éč±ø¼ņŅ×£¬ŹŹÓŚŅ°Ķā×÷Ņµ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ0112 ŌĀæ¼Ģā ĢāŠĶ£ŗŹµŃéĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ½Ī÷Ź”ÄĻ²żŅ»ÖŠÄĻ²żŹ®ÖŠ2010-2011ѧğøßČżĮŖŗĻæ¼ŹŌ ĢāŠĶ£ŗĶʶĻĢā
¹¤ŅµĮ¶ĀĮŹĒÓĆĀĮĶĮæó£ØÖ÷ŅŖ³É·ÖŹĒAl2O3£¬»¹ÓŠÉŁĮæµÄFe2O3”¢SiO2£©ĢįČ”Ņ±Į¶ĀĮµÄÖ÷ŅŖŌĮĻŃõ»ÆĀĮ£¬Č»ŗó½ųŠŠµē½ā”£¹¤ŅÕĮ÷³ĢČēĻĀĶ¼£ŗ

£Ø1£©ĀĖŅŗ1ÖŠŅŖ¼ÓČėÉŌ¹żĮæµÄŌĮĻB£¬ŌĮĻBµÄ»ÆѧŹ½ŹĒ”””””””” ”£Š“³ö²½Öč¢ŚÓŠ¹Ų·“Ó¦µÄĄė×Ó·½ ”¢ ”£
£Ø2£©Čē¹ūŹ”Č„²½Öč¢Ł£¬¼“ČܽāĀĮĶĮæó“ÓÖ±½Ó¼ÓČėŌĮĻBæŖŹ¼£¬Ōņ×īÖÕ»į¶ŌŃõ»ÆĀĮµÄÉś³ÉÓŠŹ²Ć“Ó°Ļģ£æ”””” ”£
£Ø3£©²½Öč¢ŻŹĒŌŚĀĮµē½ā²ŪÖŠ½ųŠŠ£¬µē½ā²ŪµÄĮ½¼«²ÄĮĻ¾łÓĆŹÆÄ«£¬ĘäÖŠ”” ¼«µÄ²ÄĮĻĖę·“Ó¦µÄ½ųŠŠŠčŅŖ²»¶Ļ²¹³ä£¬ĪŖŹ²Ć“£æ””””””””””
£Ø4£©ĀĮ·ŪÓėČżŃõ»Æ¶žĢś·ŪÄ©ŌŚŅżČ¼¼Į×÷ÓĆĻĀ³£ÓĆĄ“ŗø½ÓøÖ¹ģ£¬Ö÷ŅŖŹĒĄūÓĆĮĖøĆ·“Ó¦”””””” ”””””” £¬¶ųĒŅøĆ·“Ó¦ŗø½ÓĖŁ¶Čæģ”¢Éč±ø¼ņŅ×£¬ŹŹÓŚŅ°Ķā×÷Ņµ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com