
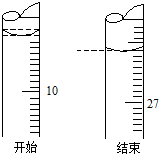
 Ä³Ń§ÉśÓūÓĆŅŃÖŖĪļÖŹµÄĮæÅØ¶ČµÄŃĪĖį²ā¶ØĪ“ÖŖĪļÖŹµÄĮæÅØ¶ČµÄĒāŃõ»ÆÄĘČÜŅŗŹ±£¬Ń”Ōń·ÓĢŖ×÷ÖøŹ¾¼Į£®ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
Ä³Ń§ÉśÓūÓĆŅŃÖŖĪļÖŹµÄĮæÅØ¶ČµÄŃĪĖį²ā¶ØĪ“ÖŖĪļÖŹµÄĮæÅØ¶ČµÄĒāŃõ»ÆÄĘČÜŅŗŹ±£¬Ń”Ōń·ÓĢŖ×÷ÖøŹ¾¼Į£®ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ·ÖĪö £Ø1£©Ėį¼īÖŠŗĶµĪ¶ØŹ±£¬ŃŪ¾¦ŅŖ×¢ŹÓ׶ŠĪĘæÄŚČÜŅŗµÄŃÕÉ«±ä»Æ£»µĪ¶ØÖÕµćŹ±ČÜŅŗŃÕÉ«ÓÉŗģÉ«Ķ»±äĪŖĪŽÉ«£¬ĒŅ°ė·ÖÖÓÄŚ²»ø“Ō£»
£Ø2£©øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©V£Ø±ź×¼£©}{V£Ø“ż²ā£©}$£¬·ÖĪö²»µ±²Ł×÷¶ŌV£Ø±ź×¼£©µÄÓ°Ļģ£¬ŅŌ“ĖÅŠ¶ĻÅØ¶ČµÄĪó²ī£»
£Ø3£©µĪ¶Ø¹ÜµÄŠ”æĢ¶ČŌŚÉĻ·½£¬×¼Č·¶ČĪŖ0.01mL£®
½ā“š ½ā£ŗ£Ø1£©ÓƱź×¼µÄŃĪĖįµĪ¶Ø“ż²āµÄĒāŃõ»ÆÄĘČÜŅŗŹ±£¬×óŹÖ°ŃĪÕĖįŹ½µĪ¶Ø¹ÜµÄ»īČū£¬ÓŅŹÖŅ”¶Æ׶ŠĪĘ棬ŃŪ¾¦×¢ŹÓ׶ŠĪĘæÖŠČÜŅŗŃÕÉ«µÄ±ä»ÆŗĶµĪ¶ØµÄĖŁ¶Č£®Ö±µ½Ņņ¼ÓČėŅ»µĪŃĪĖį£¬ČÜŅŗµÄŃÕÉ«ÓÉŗģÉ«±äĪŖĪŽÉ«£¬°ė·ÖÖÓ²»ŌŁ±äÉ«£¬Į¢¼“Ķ£Ö¹µĪ¶Ø£¬
¹Ź“š°øĪŖ£ŗ׶ŠĪĘæÖŠČÜŅŗŃÕÉ«µÄ±ä»ÆŗĶµĪ¶ØµÄĖŁ¶Č£»ŗģ£»ĪŽÉ«£»
£Ø2£©A£®ĖįŹ½µĪ¶Ø¹ÜĪ“ÓƱź×¼ŃĪĖįČÜŅŗČóĻ“¾ĶÖ±½Ó×¢Čė±ź×¼ŃĪĖįČÜŅŗ£¬±ź×¼ŅŗµÄÅضČĘ«Š”£¬Ōģ³ÉV£Ø±ź×¼£©Ę«“ó£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©V£Ø±ź×¼£©}{V£Ø“ż²ā£©}$£¬æÉÖŖc£Ø“ż²ā£©Ę«“󣬹ŹA“ķĪó£»
B£®µĪ¶ØĒ°Ź¢·ÅĒāŃõ»ÆÄĘČÜŅŗµÄ׶ŠĪĘæÓĆÕōĮóĖ®Ļ“¾»ŗóƻӊøÉŌļ£¬“ż²āŅŗµÄĪļÖŹµÄĮæ²»±ä£¬Ōģ³ÉV£Ø±ź×¼£©²»±ä£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©V£Ø±ź×¼£©}{V£Ø“ż²ā£©}$£¬æÉÖŖc£Ø“ż²ā£©²»±ä£¬¹ŹB“ķĪó£»
C£®ĖįŹ½µĪ¶Ø¹ÜŌŚµĪ¶ØĒ°ÓŠĘųÅŻ£¬µĪ¶ØŗóĘųÅŻĻūŹ§£¬Ļūŗıź×¼ŃĪĖįµÄĢå»żĘ«“ó£¬Ōģ³ÉV£Ø±ź×¼£©Ę«“ó£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©V£Ø±ź×¼£©}{V£Ø“ż²ā£©}$£¬æÉÖŖc£Ø“ż²ā£©Ę«“󣬹ŹC“ķĪó£»
D£®¶ĮČ”ŃĪĖįĢå»żŹ±£¬µĪ¶Ø½įŹųŹ±ø©ŹÓ¶ĮŹż£¬Ōģ³ÉV£Ø±ź×¼£©Ę«Š”£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©V£Ø±ź×¼£©}{V£Ø“ż²ā£©}$£¬æÉÖŖc£Ø“ż²ā£©Ę«Š”£¬¹ŹDÕżČ·£»
E£®µĪ¶Ø¹ż³ĢÖŠ£¬×¶ŠĪĘæµÄÕńµ“¹żÓŚ¼¤ĮŅ£¬Ź¹ÉŁĮæČÜŅŗ½¦³ö£¬Ōģ³ÉV£Ø±ź×¼£©Ę«Š”£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©V£Ø±ź×¼£©}{V£Ø“ż²ā£©}$£¬æÉÖŖc£Ø“ż²ā£©Ę«Š”£¬¹ŹE×¼Č·£»
¹Ź“š°øĪŖ£ŗD”¢E£»
£Ø3£©ÓÉĶ¼æÉÖŖ£¬ĘšŹ¼¶ĮŹżĪŖ9.00mL£¬ÖÕµć¶ĮŹżĪŖ26.10mL£¬¹Ź“š°øĪŖ£ŗ9.00£»26.10£®
µćĘĄ ±¾Ģāæ¼²éÖŠŗĶµĪ¶Ø£¬ĪŖøßĘµæ¼µć£¬°ŃĪÕĖį¼īÖŠŗĶµĪ¶ØµÄŹµÖŹ”¢Īó²ī·ÖĪöĪŖ½ā“šµÄ¹Ų¼ü£¬²ąÖŲ·ÖĪöÓėÓ¦ÓĆÄÜĮ¦µÄ漲飬עŅā½įŗĻ¹«Ź½·ÖĪöĪó²ī£¬ĢāÄæÄŃ¶Č²»“ó£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
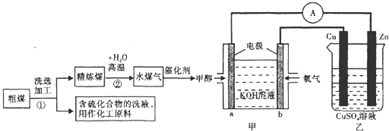
| ŹµŃé×é | ĪĀ¶Č/”ę | ĘšŹ¼Įæ/mol | Ę½ŗāĮæ/mol | “ļĘ½ŗāĖłŠčŹ±¼ä/min | ||
| C | H2O | H2 | CO | |||
| 1 | 650 | 4 | 2 | 1.6 | ” | 6 |
| 2 | 900 | 6 | 3 | ” | 1.5 | 3 |
| 3 | 900 | ” | ” | ” | ” | 1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
| ŅŅ“¼ | 1£¬2-¶žäåŅŅĶé | ŅŅĆŃ | äå | |
| דĢ¬ | ĪŽÉ«ŅŗĢå | ĪŽÉ«ŅŗĢå | ĪŽÉ«ŅŗĢå | ŗģ×ŲÉ«ŅŗĢå |
| ĆÜ¶Č£Øg/mL£© | 0.79 | 2.18 | 0.71 | 3.10 |
| ·Šµć/”ę | 78.5 | 131.4 | 34.6 | 58.8 |
| ČŪµć/”ę | -114.3 | 9.79 | -116.2 | -7.2 |
| Ė®ČÜŠŌ | »ģČÜ | ÄŃČÜ | Ī¢ČÜ | æÉČÜ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŅŅĻ©”śŅŅ¶ž“¼£ŗCH2ØTCH2$\stackrel{¼Ó³É}{”ś}$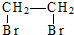 $\stackrel{Č”“ś}{”ś}$ $\stackrel{Č”“ś}{”ś}$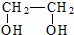 | |
| B£® | äåŅŅĶ锜ŅŅ“¼£ŗCH3CH2Br$\stackrel{ĻūČ„}{”ś}$CH2ØTCH2$\stackrel{¼Ó³É}{”ś}$CH3CH2OH | |
| C£® | 1-ä嶔Ķ锜1£¬3-¶”¶žĻ©£ŗCH3CH2CH2CH2Br$\stackrel{ĻūČ„}{”ś}$CH3CH2CH=CH2$\stackrel{¼Ó³É}{”ś}$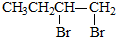 $\stackrel{ĻūČ„}{”ś}$CH2ØTCH-CHØTCH2 $\stackrel{ĻūČ„}{”ś}$CH2ØTCH-CHØTCH2 | |
| D£® | ŅŅĻ©”śŅŅČ²£ŗCH2ØTCH2$\stackrel{¼Ó³É}{”ś}$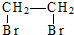 $\stackrel{ĻūČ„}{”ś}$CH”ŌCH $\stackrel{ĻūČ„}{”ś}$CH”ŌCH |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| ŹµŃé | ĪĀ¶Č/”ę | ĘšŹ¼Ź± | Ę½ŗāŹ± | |||
| n£ØCO£©/mol | n£ØH2S£©/mol | n£ØCOS£©/mol | n£ØH2£©/mol | n£ØCO£©/mol | ||
| 1 | 150 | 10.0 | 10.0 | 0 | 0 | 7.0 |
| 2 | 150 | 7.0 | 8.0 | 2.0 | 4.5 | a |
| 3 | 400 | 20.0 | 20.0 | 0 | 0 | 16.0 |
| A£® | ÉĻŹö·“Ó¦ŹĒĪüČČ·“Ó¦ | |
| B£® | ŹµŃé1 “ļĘ½ŗāŹ±£¬CO µÄ×Ŗ»ÆĀŹĪŖ70% | |
| C£® | ŹµŃé2 “ļĘ½ŗāŹ±£¬a£¼7.0 | |
| D£® | ŹµŃé3 “ļĘ½ŗāŗó£¬ŌŁ³äČė1.0molH2£¬K ÖµŌö“ó£¬Ę½ŗāÄęĻņŅĘ¶Æ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | µŚ¶ž“ĪĘ½ŗāŹ±£¬ZµÄÅضČĪŖ0.2 mol•L-1 | |
| B£® | m=3 | |
| C£® | XÓėYµÄĘ½ŗā×Ŗ»ÆĀŹÖ®±ČĪŖ1£ŗ2 | |
| D£® | ¼ÓČėZŗóĘ½ŗāĆ»ŅĘ¶Æ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | MnO2 | B£® | CO2 | C£® | H2O2 | D£® | æÕĘų |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | pH=6µÄČÜŅŗŅ»¶Ø³ŹĖįŠŌ | |
| B£® | c£ØH+£©Ė®µēĄė²śÉś=c£ØOH-£©Ė®µēĄė²śÉśµÄČÜŅŗŅ»¶Ø³ŹÖŠŠŌ | |
| C£® | Ź¹ŹÆČļŹŌŅŗĻŌŗģÉ«µÄČÜŅŗŅ»¶Ø³ŹĖįŠŌ | |
| D£® | ĒæĖįŗĶĒæ¼īµČĪļÖŹµÄĮæ»ģŗĻŗóČÜŅŗŅ»¶Ø³ŹÖŠŠŌ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com