
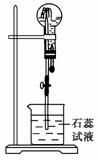
 ����A��һ�ְ�ɫ���壬����ŨNaOH��Һ���ȣ��ų���ɫ����B����Բ����ƿ�ռ������B������ͼ��ʾװ����������ѹ�ιܵĽ�ͷʱ�����Եõ���ɫ��Ȫ��A��Ũ���ᷴӦ���ų���ɫ����C����Բ����ƿ�ռ������C������ͼ��ʾװ����������ѹ�ιܵĽ�ͷʱ�����Եõ���ɫ��Ȫ��
����A��һ�ְ�ɫ���壬����ŨNaOH��Һ���ȣ��ų���ɫ����B����Բ����ƿ�ռ������B������ͼ��ʾװ����������ѹ�ιܵĽ�ͷʱ�����Եõ���ɫ��Ȫ��A��Ũ���ᷴӦ���ų���ɫ����C����Բ����ƿ�ռ������C������ͼ��ʾװ����������ѹ�ιܵĽ�ͷʱ�����Եõ���ɫ��Ȫ��
(1)A�Ļ�ѧʽ��________��
(2)�����ڳ�ȥB��ˮ�ֵĸ������______���ռ�����B�ķ�����____________��
(3)�ռ�����C�ķ�����______________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Na2O2���뵽����Al3����Mg2����NH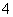 �Ļ��Һ�в��ȣ�������������������ʵ���(mol)�����Na2O2�����ʵ���(mol)�Ĺ�ϵ��ͼ��ʾ����ԭ��Һ��Al3����Mg2����NH
�Ļ��Һ�в��ȣ�������������������ʵ���(mol)�����Na2O2�����ʵ���(mol)�Ĺ�ϵ��ͼ��ʾ����ԭ��Һ��Al3����Mg2����NH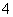 �����ʵ����ֱ�Ϊ (����)
�����ʵ����ֱ�Ϊ (����)

A��2 mol��3 mol��8 mol ������ B��3 mol��2 mol��8 mol
C��2 mol��3 mol��4 mol D��3 mol��2 mol��4 mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������е���ת��Ϊ���Ļ�����Ĺ��̳�Ϊ�̵������й�������ʵ���˹��̵�����(����)
A�����硡���������������� B����ⱥ��ʳ��ˮ
C�������̵� D�������ϳɰ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʯ���ǹ�ҵ��ѪҺ����Ҫ���ɶ�������ɵĻ���
(1)ͨ�����ں���һ������ʯ�ͻ�����չˮƽ�ı�־��________��
A��ʯ�͵IJ��� B����ϩ�IJ���
C����Ȼ���IJ��� D�����͵IJ���
(2)��ʯ�ͷ���ɵõ��۷е�����________��ʯ���������͡�ú�͡����͡�ʯ��������ȶ��ֲ�Ʒ����ҵ�ϻ����ϩ����Ҫ������________��
(3)ʯ����һ�ִ�ʯ�ͷ����еõ��Ĺ��壬������Ư����ʯ��ȼ��ʱ�����HCl���壬��������ʯ��Ư��ʱ������������________��
A���ӳɷ�Ӧ B��ȡ����Ӧ
C��ȼ�շ�Ӧ D�������ܽ�
(4)ʯ��ȼ�ջ�����Ļ�����Ⱦ������Ҫ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ����(˫ѡ)(����)
A��NH3����ˮ��ˮ��Һ�к��е�Ԫ�ص�������Ҫ��NH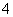
B��NH3�ڴ�������������O2��Ӧ����NO2
C��������ζ���������ƹ��Ȳ���NH3
D��̼������ȷֽ�������徭��ʯ�Ҹ����ɵô�����NH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ����H2��N2ֱ�Ӻϳɰ���N2��3H22NH3�����д�ʩ�϶���ʹ�ϳɰ���Ӧ���ʼӿ����(����)
�������¶ȡ��ڼ�����������������Ӧ��Ũ��
������ѹǿ
A��ֻ�Т٢ڢ� B��ֻ�Т٢ڢ�
C��ֻ�Т٢ۢ� D���٢ڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����(Ni—Cd)�ɳ�������ִ��������й㷺Ӧ�á���֪ij���ӵ�صĵ������ҺΪKOH��Һ����䡢�ŵ簴��ʽ���У�
Cd��2NiOOH��2H2O Cd(OH)2��2Ni(OH)2
Cd(OH)2��2Ni(OH)2
�йظõ�ص�˵����ȷ����(����)
A�����ʱ������Ӧ��Ni(OH)2��e����OH����NiOOH��H2O
B���������ǻ�ѧ��ת��Ϊ���ܵĹ���
C���ŵ�ʱ����������Һ�ļ��Բ���
D���ŵ�ʱ�������Һ�е�OH���������ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������У�������ѧ��Ӧ����ʹ��ˮ��ɫ������ʹ���Ը��������Һ��ɫ����(����)
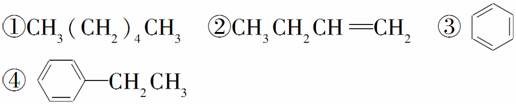
A���� B���ۢ�
C���٢� D���ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ǿ������Һ�У������������ܴ���������ǣ� ��
A��Na����Al3����NO ��Cl�� B��K����Na����Cl����AlO
��Cl�� B��K����Na����Cl����AlO
C��Fe2����K����NO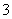 ��Cl�� D��Na����HCO
��Cl�� D��Na����HCO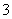 ��K����NO
��K����NO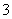
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com