
 ʵ������ȡ�����м��ַ�����
ʵ������ȡ�����м��ַ�����
| ||
| ||
| ||
| ||



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


| ||
| ||
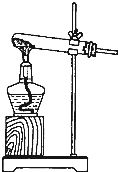
 �ڰ�����ʹʪ��ĺ�ɫ��ʯ����ֽ������ԭ���÷���ʽ��ʾ��
�ڰ�����ʹʪ��ĺ�ɫ��ʯ����ֽ������ԭ���÷���ʽ��ʾ��
| ||
| �� |
| ||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ����� | ʵ������ |
| ��Һ�ȱ�����ɫ | |
| �а�ɫ�������� | |
| �����ݲ�������ˮ��ɫ��dz |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1�������Ǹ�С��ͬѧ�����ʵ�����Ʊ������ļ��ַ�����
A.����粒������������ƹ��干��
B.���ȷֽ�NH4Cl����
C.��Ũ��ˮ��μӵ����Ƶ���ʯ����
����Ϊ���н�Ϊ������еķ�����_______________����д��ĸ�����䷴Ӧ�Ļ�ѧ����ʽΪ__________________________________��
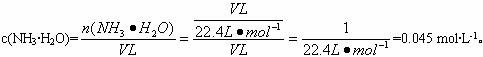
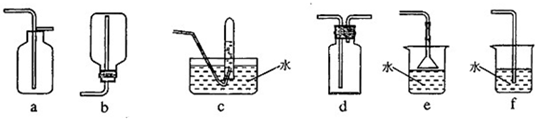
�����ͼ��������ѡ����ȡ�����ķ���װ�ã�Ҫ�������١�������__________��д��ţ���
��2����С�����ռ������İ���������ѡ������ͼ��ʾ��װ�ã�������Ӧ�ɵ��ܿ�_______���X����Y�������루����ƿ���ܵߵ�����
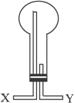
��������Һ�������ռ���������ѡ�õ��Լ���_______������ĸ��
A.H2O B.ŨH2SO4 C.CCl4 D.NaCl������Һ
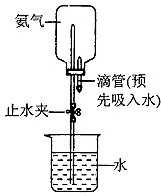
(3)��ͼʾװ�ý�����Ȫʵ�飬�ϲ���ƿ��װ�����ﰱ��������ˮ����IJ�����___________
________________________��
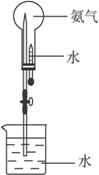
��4����ԭ��ƿ�а����dz����ģ�������Ȫʵ�����ƿ����Һ�����ʵ����ʵ���Ũ��Ϊ_________���ٶ��ڱ�״���£���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�о���ѧϰС��������Ʒ�������������ƣ�
��1�������Ǹ�С��ͬѧ�����ʵ�����Ʊ������ļ��ַ�����
A.����粒������������ƹ��干��
B.���ȷֽ�NH4Cl����
C.��Ũ��ˮ��μӵ����Ƶ���ʯ����
����Ϊ���н�Ϊ������еķ�����_______________����д��ĸ�����䷴Ӧ�Ļ�ѧ����ʽΪ__________________________________��

�����ͼ��������ѡ����ȡ�����ķ���װ�ã�Ҫ�������١�������__________��д��ţ���
��2����С�����ռ������İ�����
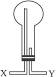
����ѡ������ͼ��ʾ��װ�ã�������Ӧ�ɵ��ܿ�_______���X����Y�������루����ƿ���ܵߵ�����
��������Һ�������ռ���������ѡ�õ��Լ���_______������ĸ��
A.H2O B.ŨH2SO4 C.CCl4 D.NaCl������Һ
(3)��ͼʾװ�ý�����Ȫʵ�飬�ϲ���ƿ��װ�����ﰱ��������ˮ����IJ�����___________
________________________��
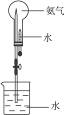
��4����ԭ��ƿ�а����dz����ģ�������Ȫʵ�����ƿ����Һ�����ʵ����ʵ���Ũ��Ϊ_________���ٶ��ڱ�״���£���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com