
���û�ѧ��Ӧԭ��֪ʶ�ش������й�̼��̼�Ļ���������⣺
(1)����β������Ҫ��Ⱦ����NO�Լ�ȼ��ȼ�ղ���ȫ��������CO���������ִ������е���Ҫ������Ⱦ�Ϊ�˼�������β����ɵĴ�����Ⱦ�����ǿ�ʼ̽������NO��CO��һ��������ת��Ϊ����������E��F�ķ���(��֪�÷�Ӧ��H<0). ��2 L�ܱ������м���һ����NO��CO�����¶ȷֱ���T1��T2ʱ����ø�����ƽ��ʱ���ʵ������±���
T/�� n/mol | NO | CO | E | F |
��ʼ | 0.100 | 0.100 | 0 | 0 |
T1 | 0.020 | 0.020 | 0.080 | 0.040 |
T2 | 0.010 | 0.010 | 0.090 | 0.045 |
�������ϱ����ݣ�д��NO��CO��Ӧ�Ļ�ѧ����ʽ .
��������ӦT1��ʱ��ƽ�ⳣ��ΪK1��T2��ʱ��ƽ�ⳣ��ΪK2�����ݱ������ݼ���K1= �����ݱ��������жϣ��¶�T1��T2�Ĺ�ϵ��(�����)__________��
A��T1>T2B��T1<T2 C��T1=T2 D�����Ƚ�
(2)��Ӧ���ɵ�����E�������������������գ�����0.4molE������200mL 3mol/L NaOH��Һ������ȫ���գ���Һ������Ũ���ɴ�С��˳��Ϊ�� .
(3)��֪CH4��H2��CO��ȼ���ȷֱ�Ϊ890.3kJ/mol��285.8 kJ/mol��283.0 kJ/mol����ҵ��������Ȼ��(��Ҫ�ɷ���CH4)��CO2���и��������Ʊ�CO��H2��д���÷�Ӧ���Ȼ�ѧ����ʽ�� .
(4)CO����������ȼ�ϵ�ص�ȼ�ϣ�ij������ȼ�ϵ�ؾ��иߵķ���Ч�ʣ�����ܵ����ӣ��õ���� Li2CO3 �� Na2CO3 �������λ����������ʣ�COΪ����ȼ����������CO2 �Ļ����Ϊ������ȼ�����Ƶ��� 650 ���¹�����ȼ�ϵ�أ�д���为���������缫��Ӧ����ʽ�������� �������� .
��֪ʶ�㡿��ѧƽ���ƶ������㡢�Ȼ�ѧ����ʽ��д����˹���ɡ��缫��Ӧʽ����д
 ���𰸽����� ��1����2CO+2NO N2+2CO2��2�֣�
���𰸽����� ��1����2CO+2NO N2+2CO2��2�֣�
��3200 L/mol����3200����2�֣� A��2�֣�
��C(Na+)>C(HCO3-)>C(CO32-)>C(OH -)>C(H+)��2�֣�
(3) CH4��g��+CO2��g��=2CO��g��+2H2��g�� ��H=+247.3 kJ•mol -1��2�֣�
��2CO+2CO32--4e-=4CO2��2�֣�O2+2CO2+4e-=2CO32-��2�֣�
��������1����NO��CO��һ��������ת��Ϊ����������E��F���ǵ����Ͷ�����̼����
 Ӧ�ķ���ʽΪ2CO+2NO N2+2CO2��ͨ��ƽ��ʱ�����ʵ����ʵ�
Ӧ�ķ���ʽΪ2CO+2NO N2+2CO2��ͨ��ƽ��ʱ�����ʵ����ʵ�
 ��Ҳ��֤��һ��Ӧ�������á�����ʽ�����м��㣺2CO + 2NO N2 + 2CO2
��Ҳ��֤��һ��Ӧ�������á�����ʽ�����м��㣺2CO + 2NO N2 + 2CO2
ʼ̬Ũ�ȣ�mol/L��0.05 0.05 0 0
�仯����mol/L�� 0.04 0.04 0.02 0.04
ƽ��Ũ�ȣ�mol/L�� 0.01 0.01 0.02 0.04
��K=C(N2)·C2(CO2)/ C2(CO)·C2(NO)=3200 L/mol��
�÷�Ӧ���ȣ��ӱ��п�������T1��T2��ƽ�������ƶ��������¶ȣ���A��T1>T2��
��2������E��CO2��0.4mol CO2����200mL 3mol/L NaOH��Һ�����ʵ�����0.6mol��������ȫ���գ���������0.2molNa2CO3��0.2molNaHCO3��CO32-��HCO3-Ҫˮ��ʹ��Һ�ʼ��ԣ�ǰ�ߵ�ˮ��̶�Զ���ں��ߣ���������Ũ�ȴ�С��
C(Na+)>C(HCO3-)>C(CO32-)>C(OH -)>C(H+)
(3) ����CH4��H2����CO��ȼ���ȿ�֪��������ȼ�յ��Ȼ�ѧ����ʽ��
��O2��g��+2H2��g��=2H2O��L����H=-571.6kJ•mol -1��
��CH4��g��+2O2��g��=CO2��g��+2H2O��L����H=-890.3kJ•mol-1��
��2CO��g��+O2��g��=2CO2��g����H=-566.0kJ•mol-1��
���ø�˹���ɽ���-��-�ۿɵã�CH4��g��+CO2��g��=2CO��g��+2H2��g����H=+247. 3 kJ•mol -1��
��4����ȼ�ϳ��У���������CO2����ʧȥ���ӵ�������Ӧ���缫��ӦΪ��O2+2CO2+4e-=2CO32-����������CO����ʧ���ӵ�������Ӧ����Ӧʽ��2CO+2CO32--4e-=4CO2����ת�Ƶ���һ���������£�������Ӧ��ӵõ��ܷ�Ӧ��2CO+O2=2CO2��
��˼·�㲦�����⿼��Ƚ��ۺϣ����淴Ӧƽ�ⳣ���ļ��㷽������˹���ɵ�Ӧ�á�ע����д�Ȼ�ѧ����ʽ��Ҫ�죻�缫��Ӧʽ����дҪ��������е����Ӵ�����ʽ���Ѷ��еȡ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ǻ�ѧ�о��г��õ�һ��˼ά��ʽ�������й����ӷ�Ӧ����ʽ��������ȷ���� (����)
| ��֪ | ���� | |
| A | ����������Һ��ͨCO2���壺Ca2����2ClO����CO2��H2O=CaCO3����2HClO | ����������Һ��ͨSO2���壺Ca2����2ClO����SO2��H2O=CaSO3����2HClO |
| B | �ö��Ե缫�������ͭ��Һ��2Cu2����2H2O | ��ͭ�缫�������ͭ��Һ��2Cu2����2H2O |
| C | ϡ������Ba(OH)2��Һ��Ӧ��pH��7ʱ��2H����SO | ����������Һ��Ba(OH)2��Һ��Ӧ��pH��7ʱ��2H����SO |
| D | Ba(OH)2��Һ��ε�������������Һ�����������ﵽ���ֵ��2Ba2����4OH����Al3����2SO42-=2BaSO4����AlO2-��2H2O | Ba(OH)2��Һ��ε������������Һ�����������ﵽ���ֵ��2Ba2����4OH����Al3����2SO42- =2BaSO4����AlO2- ��2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ư����һ�ֳ��õ���������
(1)��ҵ������Ư�۷�Ӧ�Ļ�ѧ����ʽΪ__________________________________________
________________________________________________________________________��
Ư�۵���Ч�ɷ�Ϊ________��
(2)ij̽��С����г��Ϲ�����һ����װ�����Ư�ۣ��Ը�Ư�۵ijɷֽ���̽�������������Լ������ʵ�鷽��������ʵ�顣���ڴ�������ʵ�鱨�档
��ѡ�Լ���2 mol·L��1 NaOH��Һ ��2 mol·L��1 HCl��Һ��2 mol·L��1 HNO3��Һ��0.5 mol·L��1 BaCl2��Һ��0.01 mol·L��1 AgNO3��Һ������ʯ��ˮ��ʯ����Һ����̪��Һ������ˮ��
��2 mol·L��1 HCl��Һ��2 mol·L��1 HNO3��Һ��0.5 mol·L��1 BaCl2��Һ��0.01 mol·L��1 AgNO3��Һ������ʯ��ˮ��ʯ����Һ����̪��Һ������ˮ��
| ʵ�鲽�� | Ԥ����������� |
| ����1��ȡ����Ư��������������ˮ����ֽ��裬���ã����ˣ��ó�������Һ�� | |
| ����2���������������2 mol·L��1 HCl��Һ��������������ͨ��________________________________________________________________________ ________________________________________________________________________ | ����________________________________________________________________________ ���ۣ�________________________________________________________________________ |
| ����3��ȡ��Һ��װA��B��֧�Թܡ���A�Թܣ�________________________________________________________________________ | ������Һ�ȱ��ɫ��Ȼ����ɫ�� ���ۣ�________________________________________________________________________ |
| ����4����B�Թܣ�________________________________________________________________________ ________________________________________________________________________ | ��������ɫ������ ���ۣ�________________________________________________________________________ |
(3)̽��С��Ϊ�ⶨƯ����Ca(ClO)2�ĺ�������ȡƯ��b g��ˮ�ܽ�����Ƴ�100 mL��Һ��ȷ��ȡ25.00 mL����ƿ���������������KI��Һ����ַ�Ӧ����Һ�е��������0.100 0 mol/L��Na2S2O3��Һ�ζ����ζ�2�Σ�ƽ������Na2S2O3��Һ20.00 mL�����Ư����Ca(ClO)2����������Ϊ________________________________________________________________________��
(ֻ����ʽ���������㣬��֪��Mr[Ca(ClO)2]��143��Ca(ClO)2��4HCl===2Cl2����CaCl2��2H2O��2Na2S2O3��I2===Na2S4O6��2NaI)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ȼ��0.1 mol������̬���Ļ�������3.58 L CO2(��״��)��3.6 g H2O������������(����)
A��һ���м��� B��һ��������
C��һ������ D��һ���б���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
CO2��CH4��������Ҫ���������壬ͨ��CH4��CO2��Ӧ�������ֵ��ѧƷ��Ŀǰ���о�Ŀ�ꡣ
��1��250��ʱ�������Ͻ�Ϊ��������4 L������ͨ��6 mol CO2��6 mol CH4���������·�Ӧ��CO2 (g)��CH4(g) 2CO(g)��2H2(g)��ƽ����ϵ�и��������������±���
2CO(g)��2H2(g)��ƽ����ϵ�и��������������±���
| ���� | CH4 | CO2 | CO | H2 |
| ������� | 0.1 | 0.1 | 0.4 | 0.4 |
�ٴ��¶��¸÷�Ӧ��ƽ�ⳣ��K=__________
����֪��CH4(g)��2O2(g)��CO2(g)��2H2O(g) ��H=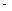 890.3 kJ·mol��1
890.3 kJ·mol��1
CO(g)��H2O (g)��CO2(g)��H2 (g) ��H=+2.8 kJ·mol��1
2CO(g)��O2(g)��2CO2(g) ��H=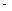 566.0 kJ·mol��1
566.0 kJ·mol��1
��ӦCO2(g)��CH4(g) 2CO(g)��2H2(g) �ġ�H=________________
2CO(g)��2H2(g) �ġ�H=________________
��2���Զ������ѱ��渲��Cu2Al2O4Ϊ���������Խ�CO2��CH4ֱ��ת�������ᡣ
���ڲ�ͬ�¶��´����Ĵ�Ч���������������������ͼ��ʾ��250��300��ʱ���¶����߶�������������ʽ��͵�ԭ����_____________________
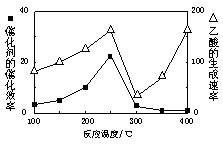
��Ϊ����߸÷�Ӧ��CH4��ת���ʣ����Բ�ȡ�Ĵ�ʩ��________________________
�۽�Cu2Al2O4�ܽ���ϡ�����е����ӷ���ʽΪ___________________________
��3��Li2O��Na2O��MgO��������CO2�������Ѱ������CO2���������ʣ����н����������______
a.���ڼ�����������Ѱ��
b.���ڢ�A����A��Ԫ���γɵ���������Ѱ��
c.���ھ���ǿ�����Ե�������Ѱ��
��Li2O����CO2�������ںϳ�Li4SiO4��Li4SiO4�������ա��ͷ�CO2��ԭ���ǣ���500�棬CO2��Li4SiO4�Ӵ�������Li2CO3��ƽ��������700�棬��Ӧ������У��ų�CO2��Li4SiO4������˵����ԭ���Ļ�ѧ����ʽ��___________________________
��4�����÷�ӦA�ɽ��ͷŵ�CO2ת��Ϊ���й�ҵ���ü�ֵ�IJ�Ʒ��
��ӦA��
���µ�⼼���ܸ�Чʵ�֣�3���з�ӦA������ԭ��ʾ��ͼ���£�
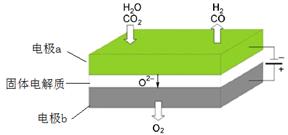
CO2�ڵ缫a�ŵ�ķ�Ӧʽ��_____________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������̿������ɵ��ȥ�������ϳɹ�ҵ�Ĵ�������������������ҵ���´ɹ�ҵ����ɫ������ɫ�����������ȡ�
��(1)п—�̼��Ե�ؾ��������ŵ��������ص㣬����õ��㷺Ӧ�á���ص��ܷ�ӦʽΪZn(s)��2MnO2(s)��H2O(l)===Zn(OH)2(s)��Mn2O3(s)��
��ص�������ӦʽΪ________________________________
��ij��ѧ�о���ѧϰС����ӷϾɸɵ���л��ն���������ȡ̼���̡�
�ٽ��ɵ�����С���ѡ�õ���ɫ������Ҫ�ɷ�ΪMnO2��ϴ�ӡ����ˡ���ɡ�
�ڽ��������尴��Һ�����2:9����Ũ���ᡢ���ȣ���Ӧ��ȫ����ˡ�Ũ����
����������Һ�м���Na2CO3��Һ���ӱ߽��裬�ٹ��˼��ɵõ�̼���̡�
��2���ڵڢڲ��У�������������Ũ�����ϵ�Ŀ����________________________________
��3��������Ϊ�����ڢ۲��е�Na2CO3��Һ����NH4HCO3��Һ��Ҳ�ܴﵽ����Ŀ�ģ���ͬʱ���������ɡ���д������NH4HCO3��Һʱ���������ӷ�Ӧ����ʽ��
_______________________________________
�� ʪ�����̼������������Һ��������̵��������̷�Ϊ���Ͻ�ȡ�������������������ӡ���Ʒ���ա������������������£�
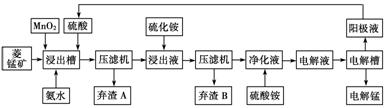
��֪�������̿�����Ҫ�ɷ���̼���̣���Ҫ������Fe2����Co2����Ni2����
���������������pH���±�������ij����Ũ��С�ڵ���10-5mol/L������Ϊ��ȫ������
| ���� | Fe(OH)2 | Ni(OH)2 | Co(OH)2 | Mn(OH)2 |
| ��ʼ����pH | 7.5 | 7.7 | 7.6 | 8.3 |
| ��ȫ����pH | 9.7 | 8.4 | 8.2 | 9.8 |
��������������������pKsp����(pKsp����lgKsp)��
| �������� | Fe(OH)3[��Դ��ȫ,Ʒ����&��*��+��] | Ni(OH)2 | Co(OH)2 | NiS | CoS |
| pKsp | 38.55 | 15.26 | 14.7 | 18.49 | 20.40 |
��4�����̿�������ᷴӦ�Ļ�ѧ����ʽ��________________________________��
��5��ʹ�ð�ˮ��Ŀ���ǵ���pHֵ��________֮��(��pH��Χ����ȷ��С�����1λ����)��
�ڡ�����Һ���м���(NH4)2S����泥���Ŀ����_________________________________
��6��������ʹ�õ��Ƕ��Ե缫�壬��ⷴӦ����ʽΪ_________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��չ��϶�������ʵʩ���ܼ��ŵ���Ҫ��ʩ֮һ����϶������ĵ綯��Ŀǰһ��ʹ�õ��������ء��������»����ʱ���綯���ṩ�ƶ��������������͵����ģ���ɲ��������ʱ�綯�����ڳ��״̬�Խ�ʡ�ܺġ������ز������Ļ�����Ϊ�����������������M��ʾ��Ϊ��������Һ����ҪΪKOH��Ϊ���Һ�������س�ŵ�ԭ����ͼ�����ܷ�ӦʽΪ�� H2+2NiOOH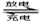 2Ni(OH)2
2Ni(OH)2
�����йػ�϶��������ж���ȷ���ǣ�������
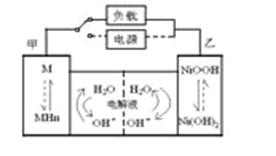
A.�����»����ʱ��ÿ����22.4LH2���ӵ缫������缫�ҵĵ�����2mol
B�������»����ʱ���ҵ缫��Χ��Һ��pH����С
C����ɲ��������ʱ����Һ�е�K+���ҵ缫Ǩ��
D����ɲ��������ʱ���缫�ĵ缫��ӦʽΪ2H2O+2e-=H2��+2OH-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ͼ��ʾ�������жϴ������(����)
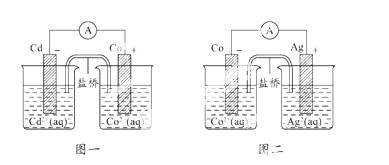
A����ԭ�ԣ�Co>Cd>Ag
B����ԭ�ԣ�Ag>Co>Cd
C �������ԣ�Co2��>Cd2��>Ag��
�������ԣ�Co2��>Cd2��>Ag��
D�������ԣ�Ag��>Co2��>Cd2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ�У���Ӧ������������ص���
A������ͨ�����ȵ�CuO��ĩ B��������̼ͨ��Na2O2��ĩ
C������Fe2O3�������� ��Ӧ D����п��Ͷ��Cu(NO3)2��Һ
��Ӧ D����п��Ͷ��Cu(NO3)2��Һ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com