
��������A��Ҫ����ҩ�����졣��һ�������£�2.30g����A��5.35gNH4Cl����ǡ����ȫ��Ӧ�����ɹ���B��4.48L����C (��״��)������C��������ˮ�õ�������Һ�������ˮB������һ�ֶ�����Ԫ�صĽ�������D������������������֪������ҵ������A���ý���D��Һ̬��C�����������·�Ӧ���Ʊ�A���ʣ�������A����Ϊ��ɫ���壬���ƵõĴ�Ʒ�����ǻ�ɫ�ģ�����A���۵�390�棬�е�430�棬�ܶȴ��ڱ���ױ�����ˮ��Ӧ���ң�ҲҪ����Ӵ��ᡢ�ƾ����ڿ�����A�����ֽ⣬�����ǿ�������ҷֽ⣬��750��800��ֽ�Ϊ������E������C��
�ش��������⣺
��1��A�Ļ�ѧʽ ��C�ĵ���ʽΪ ��
��2��A�����ᷴӦ�Ļ�ѧ����ʽΪ ��
��3��A��750��800��ֽ�ķ���ʽΪ ���ƵõĴ�Ʒ�����ǻ�ɫ�ģ�����ܵ�ԭ���� ��
��4�����õ�A���ֱܴ��ʶ�����ʹ�ã���Ҫ�������١�����������������ñ���ױ����串�ǣ�Ȼ���������ñ���ױ�ϡ������ˮ�Ҵ����Խ����仯ѧԭ����
��
��5����ҵ�Ʊ�����D������ͼ���£�

�ٲ�����в��������� ��
������ƽ���ƶ�ԭ�����Ͳ�����м�ѹ��Ŀ���� ��
��6��д��D�����⻯������ˮ��Ӧ�Ļ�ѧ����ʽΪ ��
��1��LiNH2 ��2�֣�  ��1�֣�
��1�֣�
��2��LiNH2 +2HCl =LiCl + NH4Cl��2�֣�
��3��3LiNH2 Li3 N+2NH3 ��2�֣� �ƵõIJ����к�����������1�֣�
Li3 N+2NH3 ��2�֣� �ƵõIJ����к�����������1�֣�
��4��LiNH2�ܶȴ��ڱ���ױ��Ҳ��������ǣ����Կ��ñ���ױ����и��ǣ��Ҵ��ǻ��ϵ���ϻ��ã���Ҳ���Ը�LiNH2��Ӧ������ʽΪLiNH2+C2H5OH����C2H5OLi + NH3���������ڴ��ǻ��ϵ����ˮ���ⲻ���ã��ʴ˷�Ӧ���нϻ������ɽ��������ֲ�����Σ�ա���2�֣�
��5�� ������Ũ������ȴ�ᾧ��1�֣� ��LiCl��H2O LiCl+H2O(g)��Сѹǿ������������ƽ�����������ƶ�����������ˮLiCl���Ʊ�����2�֣�
LiCl+H2O(g)��Сѹǿ������������ƽ�����������ƶ�����������ˮLiCl���Ʊ�����2�֣�
��6��LiD+H2O=LiOH+HD����2�֣�


 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ͬ����ϰ����ʦ����ѧ������ϵ�д�
ͬ����ϰ����ʦ����ѧ������ϵ�д� ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д� ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��Һ���ܺ���Cl-��SO42-��CO32-��NH4+��Fe3+��Al3+��K+��ȡ����Һ100mL���������NaOH��Һ�����ȣ��õ�0.02mol���壬ͬʱ�������ɫ���������ˣ�ϴ�ӣ����գ��õ�1.6g���壻��������Һ�м�����BaCl2��Һ���õ�4.66g����������ij������ɴ˿�֪ԭ��Һ�У� ��
A. ���ٴ���5������
B. Cl- һ�����ڣ���c(Cl‑����0.4mol/L
C. SO42-��NH4+ һ�����ڣ�Cl-���ܲ�����
D. CO32-��Al3+һ�������ڣ�K+���ܴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����е�ʵ��һ��������ֳ���������ǡ�
A��CO2����ͨ��Na2SiO3��Һ�� B��SO2����ͨ��BaCl2��Һ��
C��CO2����ͨ�뱥��Na2CO3��Һ�� D��SO2����ͨ��Ba(OH)2��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)��仯ѧ�����ս����̿�(��Ҫ�ɷ�MnO2)��Ũ�����ϼ��ȣ��������������Ƶ���������д���÷�Ӧ�����ӷ���ʽ___________________________________________
(2)���ֹ�������ˮ������ɱ����Ϊ���ƴ��ģ��Ⱦ�Լ�����������Ч����֮һ�� Ư���dz��õ�����������ҵ�Ͻ�����ͨ��ʯ����[Ca(OH)2]��ȡƯ�ۣ���ѧ��Ӧ����ʽΪ______________________________________________________
������ͼ������ֱ�߷ֱ��ʾ�ơ�ͭ��������������Cl2��Ӧʱ�����Ľ���������(����)�뷴Ӧ������������(����)�Ĺ�ϵ�����д�������Cl2��Ӧ��ֱ����________����������ʾ���ĵ��������������b��ʾ�������ֽ����е�______________
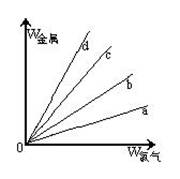
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
W��X��Y��ZΪ������Ԫ�أ�ԭ���������ε�����T��һ�ֳ����Ĺ���Ԫ�ء�
| Ԫ�� | �����Ϣ |
| W | ����Ϊ�ܶ���С������ |
| X | Ԫ����������������֮��Ϊ0 |
| Y | ��ҵ��ͨ������Һ̬��������䵥�ʣ��õ��ʵ�ij��ͬ���������DZ�������ر���������Ҫ���� |
| Z | ����������Ϊ23��������Ϊ12�ĺ��� |
| T | ��ɫ�������ʣ������Ժã������岻��ȱ�ٵ���Ԫ�أ�Ҳ����������ʹ�õĽ���֮һ |
����˵������ȷ����
A��XY2�ɹ�̬��Ϊ��̬����˷��������������Ƿ��»���
B��Y��Z�γɵ����ֻ����������һ�������¿��ת��
C��W��X��Y��T����Ԫ����ɱ�������Ԫ�ص�������Ϊ1��6��40��64�����к������������ӣ�����һ�ָ���
D��W��Z�Լ���W��Z�γɵĻ����������ǿ��ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ�����ݼ�¼��ʵ�鷽����ȷ����
A������ƽ��ȡ4.0g NaOH���壬��100mL��Ͳ����1.00 mol��L-1��NaOH��Һ
B��ʹ��25mL��ʽ�ζ��ܣ���װ�б�NaOH��Һ�����ζ�δ֪Ũ�ȵ����ᣬ��ȥNaOH��Һ22.32mL
C��ʹ��pH��ֽ���������ˮ��pHΪ4
D�����³�ѹ�£��ռ�500mLNO2���壬��NO2��������ʵ���Ϊ��0.5/22.4��mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�ʵ��ԭ���������ȷ���ǣ���������

A����10 mL��Ͳ��ȡ5.45 mL����������Һ
B���ñ�����ζ�δ֪Ũ�ȵİ�ˮ�ü�����ָʾ��
C����ͼ1װ����ȡ������������
D����ͼ2װ�÷��뱽���屽
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ۺ����ú�ˮ�����Ʊ�ʳ�Ρ��������þ�����ʣ�����������ͼ��ʾ��

��1����Ӧ�١����У�����������ԭ��Ӧ���� �� �����ţ���
��2��д����Ӧ�ڵ����ӷ���ʽ �� ��
��3��X��Һ�е���Ҫ��������Na+�� �� ��
��4�������к���Na2SO4��MgCl2��CaCl2�ȿ��������ʣ�Ϊ�Ƶô�����NaCl���壬�������£�
���ܽ⣻�����μ��������BaCl2��Һ��NaOH��Һ��Na2CO3��Һ���� �� ���ܼ���������� �� �����벹ȫȱ�ٵ�ʵ�鲽�裩
��5�����鴿����Ʒ���Ƿ�NaClӦѡ�õ��Լ��� �� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com