
��֪����A����ʽΪC3H4O2 �������ԡ�FΪ���߸�ԭ����ɵĻ�״�ṹ������ʽΪC6H8O4 ����������¿�ͼ�ش�����
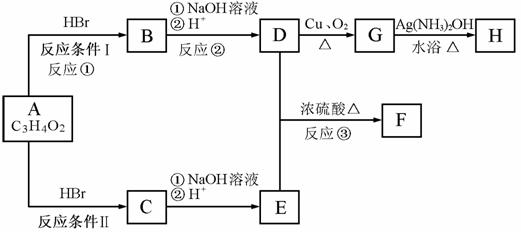
��1��A�Ľṹ��ʽΪ
��2����Ӧ�ٵķ�Ӧ����Ϊ
��3��������B�к��������ŵ�������
��4��D��E����F�Ļ�ѧ����ʽ
D��E����1:1��ӦҲ�����ɸ߾����д�����ɸø߾���Ļ�ѧ��Ӧ����ʽ��
��5��G����H�Ļ�ѧ����ʽ
��6��д��C��ͬ���칹���������������ʵĽṹ��ʽ ��
�� ������д3����
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������(C9H10O2)����ˮ����ζ������������ˮ�㾫�����쾫�ͣ���������ʳƷ��ҵ�У�Ҳ�������л��ϳ��м��塢�ܼ��ȡ����Ʊ�����Ϊ��
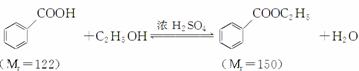
��֪��
| ��ɫ��״̬ | �е�(��) | �� �ܶ�(g��cm��3) | |
| *������ | ��ɫ��Ƭ״���� | 249 | 1.2659 |
| ���������� | ��ɫ����Һ�� | 212.6 | 1.05 |
| �Ҵ� | ��ɫ����Һ�� | 78.3 | 0.7893 |
| ������ | ��ɫ����Һ�� | 80.8 | 0.7318 |
*��������100 ���Ѹ��������
ʵ�鲽�����£�
a����100 mLԲ����ƿ�м���12.20 g�����ᡢ25 mL�Ҵ�(����)��20 mL �����飬�Լ�4 mLŨ���ᣬ��Ͼ��Ȳ������ʯ������ͼ��ʾװ�������������¶���65��70 ����Ȼ���2 h����Ӧʱ�����顪�Ҵ���ˮ���γɡ������(�е�62.6 ��)��������������÷�ˮ�����Ϸ����ȥ��Ӧ���ɵ�ˮ��������������Ҵ���
b����Ӧ�������������ų���ˮ����Һ��ر��������������ȣ�����ˮ�����ռ�����Һ�岻���������ӣ�ֹͣ���ȡ�
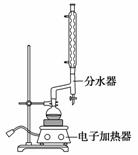
c������ƿ�ڷ�ӦҺ����ʢ������ˮ���ձ��У���������Na2CO3����Һ�����ԡ�
d���÷�Һ©���ֳ��л��㣬ˮ����25 mL������ȡ��Һ��Ȼ��ϲ����л��㡣�����Ȼ��ƣ��Դֲ�������������������Ѻ������£�����210��213�����֡�
e������ϸ�ò�Ʒ���Ϊ12.86 mL��
�ش��������⣺
(1)�ٲ���a��ʹ�÷�ˮ�����Ϸ����ȥˮ��Ŀ����
_____________________________________________________________��
�ڲ���b��Ӧ������ֵ��¶���________��
A��65��70 �桡���������� B��78��80 ��
C��85��90 �� D��215��220 ��
�ۼ����Ҵ���������Ҫԭ����____________________________________��
(2)��Na2CO3���벻�㣬�ڲ���d����ʱ��������ƿ�пɼ����������ɣ������������ԭ����__________________________________________________
______________________________________________________________��
(3)���ڲ���d�еķ�Һ����������ȷ����________��
A��ˮ��Һ�м������ѣ�ת������Һ©���У����ϲ�����������Һ©����ת������������ҡ
B����ҡ���κ����Һ©���¿ڵIJ�����������
C����������ҡ���������ֳַ�Һ©�����ô�Һ��ֲ�
D���ų�Һ��ʱ���轫�������ϵİ��۶�©�����ϵ�С��
(4)��ʵ��IJ���Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ӱ��ӵ��Ҵ���Һ�л��ձ��ӵ�ʵ���У���������������� ( )
�� ���� �� ���� �� ���÷�Һ �� ���������� �� ͨ������CO2
�� ��������NaOH��Һ �� ���������Ũ����Ļ��Һ����
A. �ܢݢ� B. �ޢ٢ݢ� C. �ޢݢ٢� D. �ߢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ˮ��Һ�������ձ���ѧ��������Ƶ�һ��ʹɳĮ�����ļ�����������ɳĮ������һ�����ľ۱�ϩ����ˮ��Һ��ˮ��Һ�еĸ߷�����ɳ����ϣ��ڵر���30-50cm���γ�һ����0.5cm�ĸ�ˮ�㣬������ֹ���µ��η����������������ˮ�����ã����жԾ۱�ϩ������˵���д�����ǣ� ( )
A�����ĵ���Ľṹ��ʽΪ��CH2=CH-COOR ��RΪ������
B������һ������������H2�����ӳɷ�Ӧ
C������һ���������ܷ���ˮ�ⷴӦ
D���ø߷��ӻ�����������ˮ��û�й̶����۷е�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��FeCl3��CuCl2��HCl�Ļ����Һ�м������ۣ�����Ӧ������ʣ��Ĺ����˳������ֹ����ܱ�������������Ӧ����Һ�д������ڵ��������ǣ� ��
A��Fe2+ B��H+ C��Cu2+��Fe2+ D��Fe3+��Cu2+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ԫ��R��X��T��Z��Q��Ԫ�����ڱ��е����λ�����±���ʾ�� ����R�����ڰ�����H2���һ��ϲ�������ը���������ж���ȷ����( )
A���ǽ����ԣ� T<X
B��R��Q�ĵ��������16
C����̬�⻯���ȶ��ԣ�R <T<Q
D������������ˮ��������ԣ�T>Q
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����йػ�ѧ�������ʽṹ��˵����ȷ����
A������Ԫ����ɵķ�����һ��ֻ�м��Լ�
B�������еļ۵��Ӷԣ������ɼ����ӶԺµ��Ӷԣ�֮�������ų�����
C���ǽ���Ԫ����ɵĻ�����һ���ǹ��ۻ�����
D�����������ӵ�����һ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1����25��ʱ����0.01mol/LNaOH��Һ�У�C(OH-)= mol/L����ˮ�������H+Ũ��= mol/L����ˮ�������OH-Ũ��= mol/L��
��2����25��ʱ����0.01mol/LHCl��Һ�У� C(H+)= mol/L����ˮ�������OH-Ũ��= mol/L,PH= ��
��3����25��ʱ��100mL0.5mol/L������͵������0.3mol/L����������Һ��Ϻ��PH= ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com