
��1����֪�����Ȼ�ѧ����ʽ��
�� H2(g)��1/2O2(g) ��H2O(g) ��H����241.8 kJ/mol
�� C(s)��1/2O2(g) ��CO(g) ��H����110.5 kJ/moL
��ˮú����Դ����ȡԭ����C(s)��H2O(g)��H2(g)��CO (g) ��H�� kJ/moL
��2����ͼ��ʾ��ѧ��Ӧ��ϵ�з�Ӧ�����������ͻ�ܵĹ�ϵ�����ϼ�ͼ�ķ���ʽΪ������������ͼ�ķ���ʽΪ����������д��A������B������C������D��ѡ�

A��CO(g)+H2O(g)=CO2(g)+H2(g) B��H2(g)+I2(g)=2HI(g)
C��H+(aq)+OH-(aq)=H2O D��CaCO3(s)=CaO(s)+CO2(g)
 ��3����ͼ��ʾ���������о�ΪCu(NO3)2��Һ���ס������ص缫���϶���������ʯī������Ӧһ��ʱ�����ش��������⣺
��3����ͼ��ʾ���������о�ΪCu(NO3)2��Һ���ס������ص缫���϶���������ʯī������Ӧһ��ʱ�����ش��������⣺
���к�ɫ�����������Ǽ׳��е�______�����ҳ��е�_______����
���ҳ��������ĵ缫��Ӧʽ��___________________________��
��4����֪25��ʱAgCl���ܶȻ�Ϊ1.77��10-10������AgCl�ı�����Һ��c(Ag+)Ϊ ������֪![]() =1.33��
=1.33��
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��Դ����������ͷ�չ����Ҫ֧�����о�����Ч�ؿ�������Դ����Դ��ȱ�Ľ��������Ҫ���������壮
��Դ����������ͷ�չ����Ҫ֧�����о�����Ч�ؿ�������Դ����Դ��ȱ�Ľ��������Ҫ���������壮| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
| 1 |
| 2 |

| 1.77 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Դ����������ͷ�չ����Ҫ֧�����о�����Ч�ؿ�������Դ����Դ��ȱ�Ľ��������Ҫ���������塣
��1����֪�����Ȼ�ѧ����ʽ��
�� H2(g)��1/2O2(g) ��H2O(g) ��H����241.8 kJ/mol
�� C(s)��1/2O2(g) ��CO(g) ��H����110.5 kJ/moL
��ˮú����Դ����ȡԭ����C(s)��H2O(g)��H2(g)��CO (g) ��H�� kJ/moL
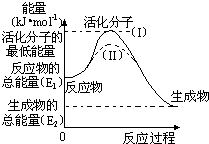
��2���о���ܶ�����Դ�����ĸ����зdz��ش��ʵ�����塣������۲���ͼ���ش����⣺
�� ͼ�з�Ӧ�Ħ�H�� kJ/mol���ú���E1��E2����ʽ��ʾ����
�� ��֪�Ȼ�ѧ����ʽ��H2(g)��1/2O2(g) �� H2O(g) ��H����241.8 kJ/mol���÷�Ӧ�Ļ��Ϊ167.2 kJ/mol�������淴Ӧ�Ļ��Ϊ kJ/mol��
�� ͼ������(II)��ʵ��(I) ����ͬһ��Ӧ���е����壺
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�����ʡ�人������·��ѧ�߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��Դ����������ͷ�չ����Ҫ֧�����о�����Ч�ؿ�������Դ����Դ��ȱ�Ľ��������Ҫ���������塣
��1����֪�����Ȼ�ѧ����ʽ��
�� H2(g)��1/2O2(g) ��H2O(g) ��H����241.8 kJ/mol
�� C(s)��1/2O2(g) ��CO(g) ��H����110.5 kJ/moL
��ˮú����Դ����ȡԭ����C(s)��H2O(g)��H2(g)��CO (g) ��H�� kJ/moL
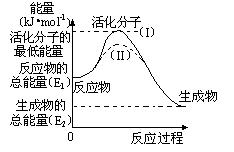
��2���о���ܶ�����Դ�����ĸ����зdz��ش��ʵ�����塣������۲���ͼ���ش����⣺
�� ͼ�з�Ӧ�Ħ�H�� kJ/mol���ú���E1��E2����ʽ��ʾ����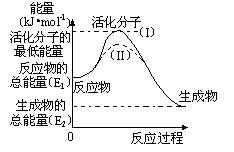
�� ��֪�Ȼ�ѧ ����ʽ��H2(g)��1/2O2(g) �� H2O(g) ��H����
����ʽ��H2(g)��1/2O2(g) �� H2O(g) ��H���� 241.8 kJ/mol���÷�Ӧ�Ļ��Ϊ167.2 kJ/mol�������淴Ӧ�Ļ��Ϊ kJ/mol��
241.8 kJ/mol���÷�Ӧ�Ļ��Ϊ167.2 kJ/mol�������淴Ӧ�Ļ��Ϊ kJ/mol��
�� ͼ������(II)��ʵ��(I) ����ͬһ��Ӧ���е����壺
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com