
| A����Һʱ��������Ȼ�̼��Һ�ӷ�Һ©���¿ڷų���ˮ����Ͽڵ��� |
| B������ʱ�����¶ȼ�ˮ�������ڱ������ʯ��Һ���£��Ҳ�����������ƿ�ĵײ� |
| C���ζ�ʱ�����ֿ��Ƶζ��ܻ����������ճ���ƿ���ߵα���ƽ�ӵζ�����Һ�� |
| D������ʱ����������ڳ���ֽ������������ƽ�����̣����������������ƽ������ |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢܢޢߢ� | B���٢ۢݢޢ� | C���٢ۢݢޢߢ� | D���٢ۢݢޢߢ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
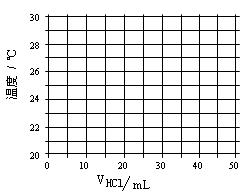
| ��������V��mL�� | 5.0 | 10.0 | 15.0 | 20.0 | 25.0 | 30.0 | 35.0 | 40.0 | 45.0 |
| NaOH�������mL) | 45.0 | 40.0 | 35.0 | 30.0 | 25.0 | 20.0 | 15.0 | 10.0 | 5.0 |
| ��Һ�¶�t���棩 | 22.2 | 23.3 | 24.6 | 25.8 | 27.0 | 27.8 | 26.1 | 24.4 | 22.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������ܼ��ķ��������Ȼ���ϡ��Һ����Ũ��Ϊ������Һ |
| B������ᴢ���ڴ�����������ɫ�Լ�ƿ�� |
| C�������ش�����ú���� |
| D��ʵ������ȡ��ϩʱ��������������CO2��SO2��H2O��g�����ʣ�������ͨ�����������Һ�ͼ�ʯ�ҵýϴ�������ϩ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������� | B������ | C��̼������Һ | D������������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

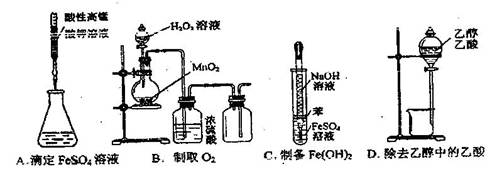
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 �����ʣ��ᴿ����·�����£�
�����ʣ��ᴿ����·�����£�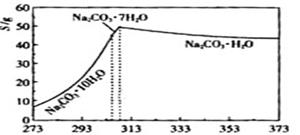
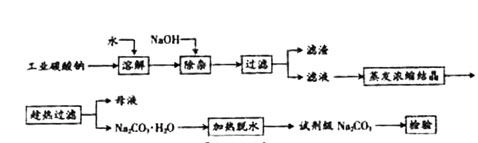
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A��������ƿ����Һת���Ƶ�����ƿ��ʱ��δϴ�ӿ�����ƿ |
| B������ʱ�����ӿ̶��� |
C������ʱ�����ӿ̶��� |
| D����Һʱ��������Һ�彦�� |
 ��֪���ζ��������漰���ķ�Ӧ����ʽ��(NH4)2 B4O7 + 2HCl + 5H2O = 2NH4Cl + 4H3BO3��
��֪���ζ��������漰���ķ�Ӧ����ʽ��(NH4)2 B4O7 + 2HCl + 5H2O = 2NH4Cl + 4H3BO3���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com