
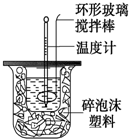
 ������ͼװ�òⶨ�кͷ�Ӧ�ķ�Ӧ�ȵ�ʵ�鲽�����£�
������ͼװ�òⶨ�кͷ�Ӧ�ķ�Ӧ�ȵ�ʵ�鲽�����£�| �¶� ʵ������� | ��ʼ�¶�t1�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 29.5 | |
| 2 | 25.9 | 25.9 | 25.9 | 29.2 | |
| 3 | 26.4 | 26.2 | 26.3 | 29.8 | |
���� ��1����NaOH��Һ����С�ձ��У����ּܷ��ε��룬����ᵼ������ɢʧ��Ӱ��ⶨ�����
��2��������������ƻ��ʱ���������¶ȼ��ϵĻ��β������������ؽ�����ʹ������NaOH��Һ��Ͼ��ȣ�
��3�����ж��¶Ȳ����Ч�ԣ�Ȼ������¶Ȳ�ƽ��ֵ���ٸ���Q=m•c•��T���㷴Ӧ�ų���������
��4��a��װ�ñ��¡�����Ч�����õ�����ƫС��
b�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶ȣ��������ʼ�¶�ƫ�ߣ��¶Ȳ�ƫС��
c����ȡNaOH��Һ�����ʱ���Ӷ������ᵼ�������������������ƫ�ų����������䣬���¶Ȳ�ƫС��
d���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У�����ɢʧ��
��� �⣺��1����������������Һʱ������һ��Ѹ�ٵĵ��룬Ŀ���Ǽ���������ɢʧ�����ּܷ��ε�������������Һ������ᵼ������ɢʧ��Ӱ��ⶨ�����
�ʴ�Ϊ��C��
��2��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ���������ǣ��������¶ȼ��ϵĻ��β������������ؽ������¶ȼ��Dz����¶ȵģ�����ʹ���¶ȼƽ��裻Ҳ������������ձ���������ܵ���Һ�彦��������ɢʧ��Ӱ��ⶨ����������ܴ�ӲֽƬ�ò��������裬�����������ɢʧ��
�ʴ�Ϊ��D��
��3��3���¶Ȳ�ֱ�Ϊ��3.4�棬3.3�棬3.5�棬����Ч���¶Ȳ�ƽ��ֵ=3.4�棻50mL0.25mol/L������50mL0.55mol/L NaOH��Һ�����кͷ�Ӧ����ˮ�����ʵ���Ϊ0.05L��0.25mol/L��2=0.025mol����Һ������Ϊ��100ml��1g/ml=100g���¶ȱ仯��ֵΪ��T=3.4�棬������0.025molˮ�ų�������ΪQ=m•c•��T=100g��4.18J/��g•�棩��3.4��=1421.2J=1.4212kJ��
�ʴ�Ϊ��1.4212��
��4��a��װ�ñ��¡�����Ч�����õ�����ƫС���к��ȵ���ֵƫС����a��ȷ��
b���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳ���ϡH2SO4���¶ȣ��������ʼ�¶�ƫ�ߣ��¶Ȳ�ƫС���к��ȵ���ֵƫС����b��ȷ��
c����ȡNaOH��Һ�����ʱ���Ӷ������ᵼ�������������������ƫ�ų����������䣬���¶Ȳ�ƫС���к��ȵ���ֵƫС����c��ȷ��
d���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У�����ɢʧ���к��ȵ���ֵƫС����d��ȷ��
�ʴ�Ϊ��abcd��
���� ������Ҫ�����к��ȵIJⶨ����Ŀ�Ѷ��еȣ���ȷ�к��ȵĸ���ⶨ�к��ȵķ������������ķ���Ϊ���ؼ�������������ѧ���ķ�����������ѧʵ��������


 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ϡ���ᡢϡH2SO4 | B�� | KCl��BaCl2��CuSO4 | ||
| C�� | KCl��BaCl2��CaCl2 | D�� | FeCl3��Fe2��SO4��3��NaCl | ||
| E�� | FeCl3��Fe2��SO4��3��NaCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������ʵ��� | B�� | �������֮���ƽ������ | ||
| C�� | ������ӱ����Ĵ�С | D�� | ���������A |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ˮ��Ӧ��Na+H2O�TNa++OH-+H2�� | |
| B�� | ����CuSO4��Һ��Ӧ��2Na+Cu2+�TCu��+2Na+ | |
| C�� | NaHCO3��Һ��ϡH2SO4��Ӧ��CO${\;}_{3}^{2-}$+2H+�TH2O+CO2�� | |
| D�� | NaOH��Һ��NaHSO4��Һ��Ӧ��H++OH-�TH2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | v��A��=30mol•L-1•min-1 | B�� | v��B��=0.3mol•L-1•s-1 | ||
| C�� | v��C��=48mol•L-1•min-1 | D�� | v��D��=1mol•L-1•s-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ҫ����Ũ��Ϊ0.25mol•L-1��NaOH��Һ480mL��Ӧ����4.8g NaOH��250mL���ձ����ܽ⣬��ȴ����ת�Ƶ�500mL����ƿ�У�ϴ�ӡ�ת�ơ����� | |
| B�� | ����NaOH��Һ�����ձ����ܽ�NaOH��δ��ȴ�����¾�ת�Ƶ�����ƿ�У���ҺŨ��ƫ�� | |
| C�� | ����һ�����ʵ���Ũ�ȵ���Һ����ʱ�����ӿ̶��ߵ�������Ũ��ƫ�� | |
| D�� | ����20g�ܶ�Ϊ��g•cm-3��Ca��NO3��2��Һ�к���2g Ca��NO3��2������Һ��NO3-�����ʵ���Ũ��Ϊ25��/41mol•L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CuO | B�� | NO2 | C�� | FeCl2 | D�� | SO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��a=b��pH��NaX����pH��NaY����������HX��HY | |
| B�� | ��a=b��c��X -��=c��Y -��+c��HY����������HX��HY | |
| C�� | ��a��b��c��X -��=c��Y -����������HX��HY | |
| D�� | ��a=0.1mol/L������Һ�������ϣ���c��X -��+c��HX��=0.1mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
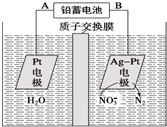
| A�� | Ǧ���ص�A��Ϊ�������缫����ΪPbO2 | |
| B�� | �õ��ص��ܷ�Ӧ����ʽΪ��2H2O+4NO3- $\frac{\underline{\;���\;}}{\;}$2N2��+5O2��+4OH- | |
| C�� | �õ��ص�������ӦʽΪ��2NO3-+12H++10e-�TN2��+6H2O | |
| D�� | ����������ת��2mol���ӣ���Ĥ�Ҳ���Һ����������5.6g |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com