
·ÖĪö £Ø1£©¢ŁĀČ»ÆĒāĪŖĒæµē½āÖŹ£¬ĶźČ«µēĄė0.1mol•L-1µÄŃĪĖįĒāĄė×ÓÅضČĪŖ0.1mol•L-1£»¢ŚĮņĖįĪŖĒæµē½āÖŹ£¬0.2mol•L-1µÄĮņĖį£¬ĒāĄė×ÓÅضČĪŖ””0.4mol•L-1£»””¢Ū“×ĖįĪŖČõµē½āÖŹ£¬²æ·ÖµēĄė£¬0.1mol•L-1µÄ“×ĖįĒāĄė×ÓÅØ¶ČŠ”ÓŚ0.1mol•L-1£»¢ÜŅ»Ė®ŗĻ°±ĪŖČõµē½āÖŹ£¬²æ·ÖµēĄė£¬0.1mol•L-1µÄ°±Ė®ĒāŃõøłĄė×ÓÅØ¶ČŠ”ÓŚ0.1mol•L-1£»””¢ŻĒāŃõ»ÆÄĘĪŖĒæµē½āÖŹ£¬ĶźČ«µēĄė£¬0.1mol•L-1µÄNaOHČÜŅŗÖŠĒāŃõøłĄė×ÓÅضČ0.1mol•L-1””¢ŽĒāŃõ»Æ±µĪŖĒæµē½āÖŹ£¬ĶźČ«µēĄė£¬0.1mol•L-1µÄBa£ØOH£©2ČÜŅŗ£¬ĒāŃõøłĄė×ÓÅضČĪŖ0.2mol•L-1£»
£Ø2£©µē½āÖŹŹĒÖø£ŗŌŚĖ®ČÜŅŗÖŠ»ņČŪȌדĢ¬ĻĀÄܹ»µ¼µēµÄ»ÆŗĻĪļ£®µē½āÖŹĖ®ČÜŅŗÖŠ»ņČŪȌדĢ¬ĻĀÄܹ»µ¼µē£¬ŹĒŅņµē½āÖŹ×ŌÉķæÉŅŌĄė½ā³É×ŌÓÉŅĘ¶ÆµÄĄė×Ó£»
·Ēµē½āÖŹŹĒÖø£ŗŌŚĖ®ČÜŅŗĄļŗĶČŪȌדĢ¬ĻĀ¶¼²»µ¼µēµÄ»ÆŗĻĪļ£»µ„ÖŹ”¢»ģŗĻĪļ¼Č²»ŹĒµē½āÖŹŅ²²»ŹĒ·Ēµē½āÖŹ£»
Ēæµē½āÖŹŹĒŌŚĖ®ČÜŅŗÖŠ»ņČŪȌדĢ¬ĻĀÄÜĶźČ«µēĄėµÄµē½āÖŹ£¬°üĄØĒæĖį”¢Ēæ¼ī”¢»īĘĆ½šŹōŃõ»ÆĪļŗĶ“ó²æ·ÖŃĪ£»
£Ø3£©¼ÓĖ®Ļ”ŹĶČõĖįŹ±£¬»į“Ł½ųČõĖįµÄµēĄė£»ŅĄ¾ŻÖŠŗĶ·“Ó¦ÖŠĻūŗĵÄĒāĄė×ÓµÄĪļÖŹµÄĮæµČÓŚĒāŃõøłĄė×ÓµÄĪļÖŹµÄĮæ½ā“š£»
£Ø4£©Éč³öĖįČÜŅŗµÄpHĪŖa£¬¼īČÜŅŗµÄpHĪŖb£¬øł¾ŻøĆĪĀ¶ČŅŌ¼°Ģå»ż¹ŲĻµĮŠŹ½¼ĘĖć£®
½ā“š ½ā£ŗ£Ø1£©£©¢ŁĀČ»ÆĒāĪŖĒæµē½āÖŹ£¬ĶźČ«µēĄė0.1mol•L-1µÄŃĪĖįĒāĄė×ÓÅضČĪŖ0.1mol•L-1£»¢ŚĮņĖįĪŖĒæµē½āÖŹ0.2mol•L-1µÄĮņĖį£¬ĒāĄė×ÓÅضČĪŖ””0.4mol•L-1£»””¢Ū“×ĖįĪŖČõµē½āÖŹ£¬²æ·ÖµēĄė£¬0.1mol•L-1µÄ“×ĖįĒāĄė×ÓÅØ¶ČŠ”ÓŚ0.1mol•L-1£»¢ÜŅ»Ė®ŗĻ°±ĪŖČõµē½āÖŹ£¬²æ·ÖµēĄė£¬0.1mol•L-1µÄ°±Ė®ĒāŃõøłĄė×ÓÅØ¶ČŠ”ÓŚ0.1mol•L-1£»””¢ŻĒāŃõ»ÆÄĘĪŖĒæµē½āÖŹ£¬ĶźČ«µēĄė£¬0.1mol•L-1µÄNaOHČÜŅŗÖŠĒāŃõøłĄė×ÓÅضČ0.1mol•L-1””¢ŽĒāŃõ»Æ±µĪŖĒæµē½āÖŹ£¬ĶźČ«µēĄė£¬0.1mol•L-1µÄBa£ØOH£©2ČÜŅŗ£¬ĒāŃõøłĄė×ÓÅضČĪŖ0.2mol•L-1£»ĒāĄė×ÓÅضČŌ½“ó£¬pHŌ½Š”£¬ĒāŃõøłĄė×ÓÅضČŌ½“ó£¬pHŌ½“ó£¬ĖłŅŌpHÓÉŠ”µ½“óµÄĖ³Šņ£ŗ¢Ś¢Ł¢Ū¢Ü¢Ż¢Ž£»¹Ź“š°øĪŖ£ŗ¢Ś¢Ł¢Ū¢Ü¢Ż¢Ž£»
£Ø2£©¢ŁNaClŹĒŃĪ£¬ČŪȌדĢ¬»ņÕßČÜÓŚĖ®Äܹ»ĶźČ«µēĄė£¬ŹōÓŚĒæµē½āÖŹ£»””¢ŚBaSO4ŹĒŃĪČŪȌדĢ¬Äܹ»ĶźČ«µēĄė£¬ŹōÓŚĒæµē½āÖŹ£»
¢ŪH2SO4ŹĒĒæĖį£¬ČÜÓŚĖ®Äܹ»ĶźČ«µēĄė£¬ŹōÓŚĒæµē½āÖŹ£»¢ÜKOHŹĒĒæ¼ī£¬ČÜÓŚĖ®»ņÕßČŪȌדĢ¬ÄÜĶźČ«µēĄėŹōÓŚĒæµē½āÖŹ£»
¢ŻŹÆÄ«ŹĒµ„ÖŹ¼Č²»ŹĒµē½āÖŹ£¬Ņ²²»ŹĒ·Ēµē½āÖŹ£»””¢ŽH2CO3ŹĒČõĖįĖ®ČÜŅŗÖŠÄܲæ·ÖµēĄė£¬ŹōÓŚČõµē½āÖŹ£»
¢ßCH3COOH””ŹĒČõĖįĖ®ČÜŅŗÖŠÄܲæ·ÖµēĄė£¬ŹōÓŚČõµē½āÖŹ£»¢ąNH3•H2O ŹĒČõ¼ī£¬Ė®ČÜŅŗÖŠÄܲæ·ÖµēĄė£¬ŹōÓŚČõµē½āÖŹ£» ¢įÕįĢĒŌŚĖ®ČÜŅŗŗĶČŪȌדĢ¬¶¼²»µ¼µē£¬ŹōÓŚ·Ēµē½āÖŹ£»””¢ā¾Ę¾«ŌŚĖ®ČÜŅŗŗĶČŪȌדĢ¬¶¼²»µ¼µē£¬ŹōÓŚ·Ēµē½āÖŹ£»⑪SO2±¾Éķ²»ÄܵēĄė£¬ŹōÓŚ·Ēµē½āÖŹ£»⑫Cu µ„ÖŹ£¬¼Č²»ŹĒµē½āÖŹŅ²²»ŹĒ·Ēµē½āÖŹ£»⑬Cl2 ŹĒµ„ÖŹ¼Č²»ŹĒµē½āÖŹ£¬Ņ²²»ŹĒ·Ēµē½āÖŹ£»⑭CO2£¬±¾Éķ²»ÄܵēĄė£¬ŹōÓŚ·Ēµē½āÖŹ£»
ŹōÓŚĒæµē½āÖŹµÄÓŠ£ŗ¢Ł¢Ś¢Ū¢Ü£»ÖŠŹōÓŚČõµē½āÖŹµÄÓŠ£ŗ¢Ž¢ß¢ą£»ĘäÖŠŹōÓŚ·Ēµē½āÖŹµÄÓŠ¢į¢ā⑪⑭£»¹Ź“š°øĪŖ£ŗ¢Ł¢Ś¢Ū¢Ü£»¢Ž¢ß¢ą£»¢į¢ā⑪⑭£»
£Ø3£©Ä³Ņ»ŌŖČõĖįČÜŅŗ£ØA£©Óė¶žŌŖĒæĖį£ØB£©µÄpHĻąµČ£¬ŌņČõĖįÓŠ“ó²æ·ÖĪŖµēĄė£¬¼ÓĖ®Ļ”ŹĶŹ±£¬Äܹ»“Ł½ųøü¶ąµÄČõĖįµēĄė£¬ĖłŅŌ¼ÓĖ®Ļ”ŹĶŗó£¬ĒāĄė×ÓÅØ¶Č“óÓŚ¶žŌŖĒæĖįÖŠĒāĄė×ÓÅØ¶Č£¬ĖłŅŌpHA£¼pHB£¬ÉĻŹöĻ”ŹĶČÜŅŗÖŠČõĖįµÄĒāĄė×ÓµÄĪļÖŹµÄĮæÅØ¶Č“óÓŚĒæĖį£¬ĖłŅŌµČĢå»żŗ¬ÓŠµÄĒāĄė×ÓµÄĪļÖŹµÄĮæ¶ą£¬ÖŠŗĶµČÅØ¶ČµČĢå»żµÄNaOHČÜŅŗ£¬ÓƵÄĢå»żÉŁ£»
¹Ź“š°øĪŖ£ŗ£¼£»£¼£»
£Ø4£©ÉčĒæĖįČÜŅŗµÄpHĪŖa£¬Ģå»żĪŖV£¬pH=-lgc£ØH+£©£¬ČÜŅŗÖŠĒāĄė×ÓÅضČĪŖ£ŗ10-amol/L£»¼īČÜŅŗµÄpHĪŖb£¬Ģå»żĪŖ10V£¬ČÜŅŗÖŠĒāŃõøłĄė×ÓµÄÅضČĪŖ£ŗc£ØOH-£©=10-£Ø14-b£©mol/L£¬»ģŗĻŗóČÜŅŗ³ŹÖŠŠŌ£¬ŌņĀś×ćČÜŅŗÖŠĒāĄė×ÓµÄĪļÖŹµÄĮæ“óÓŚĒāŃõøłĄė×ÓµÄĪļÖŹµÄĮ棬¼“10-amol/L”ĮVL=10-£Ø14-b£©mol/L”Į10VL£¬
½āµĆ£ŗ-a=b-13£¬a+b=13£¬¼“pHĖį+pH¼ī=13£¬¹Ź“š°øĪŖ£ŗpHĖį+pH¼ī=13£®
µćĘĄ ±¾Ģāæ¼²éĮĖĄė×ÓÅØ¶Č“óŠ”µÄ±Č½Ļ£¬µē½āÖŹ”¢Ēæµē½āÖŹ”¢·Ēµē½āÖŹµÄÅŠ¶Ļ£¬ČÜŅŗĖį¼īŠŌÓėpHÖµµÄĻą¹Ų¼ĘĖć£¬ĢāÄæ×ŪŗĻŠŌĒ棬Éę¼°ÄŚČŻ¶ą£¬ÄѶČÖŠµČ£¬½āĢāŹ±×¢Ņā°ŃĪÕµē½āÖŹĒæČõµÄÅŠ¶ĻŅĄ¾Ż£¬×¢ŅāČõµē½āÖŹµēĄėµÄĢŲµć£®


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĻĖĪ¬ĖŲ”¢µ°°×ÖŹ¶¼ŹĒÓŠ»śøß·Ö×Ó»ÆŗĻĪļ | |
| B£® | ±½”¢¼×±½µČŹĒĆŗµÄÖ÷ŅŖ³É·Ö | |
| C£® | »ØÉśÓĶ”¢Å£ÓĶ¶¼ŹĒõ„Ąą | |
| D£® | ¶¹½¬”¢Å£ÄĢæÉ×÷ĪŖĪóŹ³ÖŲ½šŹōŃĪµÄ½ā¶¾¼Į |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¹żŃõ»ÆÄʵĵē×ÓŹ½£ŗ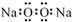 | |
| B£® | ”°ÓńĶĆ”±ŗÅŌĀĒņ³µÉĻŹ¹ÓƵÄÖŹ×ÓŹż94£¬ÖŠ×ÓŹż144µÄīŠŌ×Ó£ŗ${\;}_{94}^{144}$Pu | |
| C£® | CH4µÄĒņ¹÷Ä£ŠĶ£ŗ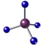 | |
| D£® | NaµÄŌ×Ó½į¹¹Ź¾ŅāĶ¼£ŗ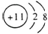 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā

| A£® | £Ø1£©ÖŠŗģ×ŪÉ«±äÉī£¬£Ø2£©ÖŠŗģ×ŲÉ«±äĒ³ | B£® | £Ø1£©ÖŠŗģ×ŪÉ«±äĒ³£¬£Ø2£©ÖŠŗģ×ŲÉ«±äÉī | ||
| C£® | ÉÕĘæ£Ø1£©ÖŠĘųĢåµÄŃ¹Ēæ²»±ä | D£® | ÉÕĘæ£Ø2£©ÖŠĘųĢåµÄŃ¹ĒæŌö“ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ·“Ó¦ | “óĘų¹ĢµŖ N2£Øg£©+O2£Øg£©?2NO£Øg£© | |
| ĪĀ¶Č/”ę | 27 | 2000 |
| K | 3.84”Į10-31 | 0.1 |
| ČŻĘ÷ ±ąŗÅ | ĘšŹ¼Ź±ø÷ĪļÖŹĪļÖŹµÄĮæ/mol | “ļĘ½ŗāŹ±ĢåĻµµÄ ÄÜĮæ±ä»Æ | ||
| N2 | H2 | NH3 | ||
| a | 1 | 3 | 0 | ·ÅČČ23.1kJ |
| b | 0.6 | 1.8 | 0.8 | ĪüČČQ kJ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 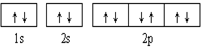 æɱķŹ¾µ„ŗĖ10µē×ÓĮ£×Ó»łĢ¬Ź±µē×ÓÅŲ¼ æɱķŹ¾µ„ŗĖ10µē×ÓĮ£×Ó»łĢ¬Ź±µē×ÓÅŲ¼ | |
| B£® | 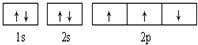 “ĖĶ¼“ķĪó£¬Ī„±³ĮĖÅŻĄūŌĄķ “ĖĶ¼“ķĪó£¬Ī„±³ĮĖÅŻĄūŌĄķ | |
| C£® | 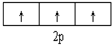 ±ķŹ¾»łĢ¬NŌ×ӵļŪµē×ÓÅŲ¼ ±ķŹ¾»łĢ¬NŌ×ӵļŪµē×ÓÅŲ¼ | |
| D£® |  ±ķŹ¾“¦ÓŚ¼¤·¢Ģ¬µÄBµÄµē×ÓÅŲ¼Ķ¼ ±ķŹ¾“¦ÓŚ¼¤·¢Ģ¬µÄBµÄµē×ÓÅŲ¼Ķ¼ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ė® | B£® | Ģ¼ĖįĒāÄĘČÜŅŗ | C£® | ŹÆČļŹŌŅŗ | D£® | ĮņĖįĒāÄĘČÜŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 øßÖŠ»Æѧ½Ģ²Ä½éÉÜĮĖÄĘ”¢Ć¾”¢ĀĮ”¢Ģś”¢ĀČ”¢Įņ”¢µŖ”¢¹čµČŌŖĖŲ¼°Ęä»ÆŗĻĪļµÄÖŖŹ¶£¬ŹĒѧĻ°ŃŠ¾æĘäĖü»ÆѧÖŖŹ¶µÄŌŲĢ壮
øßÖŠ»Æѧ½Ģ²Ä½éÉÜĮĖÄĘ”¢Ć¾”¢ĀĮ”¢Ģś”¢ĀČ”¢Įņ”¢µŖ”¢¹čµČŌŖĖŲ¼°Ęä»ÆŗĻĪļµÄÖŖŹ¶£¬ŹĒѧĻ°ŃŠ¾æĘäĖü»ÆѧÖŖŹ¶µÄŌŲĢ壮²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÉŁĮæSO2ĶØČėCa£ØClO£©2ČÜŅŗÖŠ£ŗSO2+H2O+Ca2++2ClO-ØTCaSO3”ż+2HClO | |
| B£® | Fe£ØOH£©3ČÜŅŗĒāµāĖį£ŗFe£ØOH£©3+3H+ØTFe3++3H2O | |
| C£® | ĶČÜÓŚĻ”ĻõĖį£ŗ3Cu+8H++2NO3-ØT3Cu2++2NO”ü+4H2O | |
| D£® | Na2S2O3ČÜŅŗÖŠĶØČė×ćĮæĀČĘų£ŗS2O32-+2Cl2+3H2OØT2SO32-+4Cl-+6H+ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com