
ʵ���о����֣����ᷢ��������ԭ��Ӧʱ�������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�͡�ijͬѧȡһ���������Ͻ���100mLijŨ�ȵ������ַ�Ӧ����Ӧ������������ų����ڷ�Ӧ���������Һ�У���μ���4mol��L��1������������Һ����������������Һ�����(mL)������ij��������ʵ���(mol)�Ĺ�ϵ��ͼ��ʾ��C��0����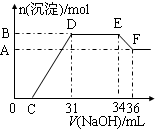
�Իش��������⣺
��1��д����Ӧ������DE�ε����ӷ�Ӧ����ʽ�� ��EF�����ɺ���Ԫ�����ӵ������� ��
��2���Ͻ��У���������Ϊ g����������Ϊ g ��
��3��C��ֵΪ mL��
��4��������Һ�����ʵ���Ũ��Ϊ mol��L��1��
��10�֣�������⣬����ÿ��2�֣���1�� NH4��+OH����NH3?H2O ��1�֣�
ƫ����������ǻ����������1�֣� ��2�� 0.216 1.344 ��3��7 ��4��1.48
���������������1��OC֮��û�г������ɣ�˵�����������OC֮�䷢���ķ�Ӧ����кͷ�Ӧ�����ӷ���ʽΪH++OH-��H2O��CD�����ڽ������������������Ƶķ�Ӧ���ֱ�����������������������������DEһ�γ�������û�з����仯��ΪNH4NO3��NaOH��Ӧ����Ӧ�����ӷ���ʽΪNH4��+OH����NH3?H2O��EF��Ϊ�������������������Ƶķ�Ӧ����Al(OH)3 +OH-��AlO2��+2H2O��Al(OH)3 +OH-��[Al(OH)4]�������EF�����ɺ���Ԫ�����ӵ�������ƫ����������ǻ����������
��2����ͼ��֪��EF�����ĵ�����������ҺΪ36mL��34m��2mL����ý��вμӷ�Ӧ����������Ϊ0.002L��4mol/L��0.008mol������Al(OH)3 +OH-��AlO2��+2H2O��֪��Al(OH)3�����ʵ���Ϊ0.008mol�������ԭ���غ��֪����������������0.008mol��27g/mol��0.216g��DE����������������Һ�������34ml��31ml��3mol������вμӷ�Ӧ����������Ϊ0.003L��4mol/L��0.012mol�����Ը��ݷ�ӦNH4��+OH����NH3?H2O��֪��NH4�������ʵ�����0.012mol�����ݵ�ԭ���غ��֪�����ɰ��������ʵ�����0.012mol�������ǻ�ԭ������Ը��ݵ��ӵ�ʧ�غ��֪���������ʵ�������0.012mol��8��0.008mol��3����3��0.024mol���������������0.024mol��56g/mol��1.344g��
��3�����ݷ�ӦʽFe3����3OH��=Fe(OH)3����Al3����3OH��=Al(OH)3����֪��C��D�������������Ƶ����ʵ�����0.024mol��3��0.008mol��3��0.096mol���������0.096mol��4mol/L��0.024L��24ml������C���Ӧ�������31ml��24ml��7ml��
��4��E���Ӧ�������������ƣ������������غ��֪�������Ƶ����ʵ�����0.034L��4mol/L��0.136mol�����Ը��ݵ�ԭ���غ��֪��ԭ��������ʵ�����0.136mol��0.012mol��0.148mol�����������Ũ����0.148mol��0.1L��1.48L��
���㣺�������������ķ�Ӧ���йؼ���


 С�����ϵ�д�
С�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ϊά������ѪҺ�е�Ѫ�Ǻ������ڸ�������Һʱ��ͨ����������ע��Һ����ͼ��ҽԺ������Һʹ�õ�һƿ��������Ϊ5%�������ǣ�C6H12O6��ע��Һ��ǩ��������۲��ǩ���������ݺ���㣺
��1������Һ�����ʵ���Ũ��Ϊ mol��L��1���������2λ��Ч���֣���
��2������Һ���ܶ�Ϊ g��mL��1��
��3������Ӹ�ƿ��ȡ��75g������ע��Һ�������Ϊ15%��ע��Һ����Ҫ���� g�����ǹ��壨��ȷ��0.1g����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)ͬ��ͬѹ�£�ͬ�����NH3��H2S�������������___________��ͬ������NH3��H2S������������__________��ͬ������NH3��H2S������������ԭ�Ӹ�������___________��������������ԭ�Ӹ�����ȣ����ǵ����ʵ�������________��
(2)Na2SO4??10H2O��Ħ��������__________��483gNa2SO4??10H2O������Na2SO4??10H2O�����ʵ�����_______������Na�������ʵ�����_________������H2O���ӵ���Ŀ��_______����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ��пƬ��������CuSO4��Һ1L�У����Թ۲쵽пƬ�����л�ɫ������������������ʱпƬ����������0.1g����������Һ��仯���Բ��ƣ�����
��1����1���μӷ�Ӧ�Ļ�ԭ�������ʵ����� ��
��2����ԭ��������ʵ����� ��
��3������ZnSO4�����ʵ���Ũ���� ��
��4����Һ������ ��������ӡ����١���
��5��д���÷�Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ڻ�ƿ�м��롰�ʻ����ʼ��������ӳ��ʻ����������±���500mL���ʻ����ʼ�����Һ�к��еijɷ֣��Ķ���ش��������⣺
| �ɷ� | ������g�� | Ħ��������g ��mol��1�� |
| ���� | 25.0 | 342 |
| ����� | 0.3 | 174 |
| ��˾ƥ�� | 0.2 | 180 |
| ������� | 0.3 | 158 |
| ������ | 0.1 | 170 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����֪10g CaCO3�ֽ���Ҫ����17.56kJ ��������д��̼��Ʒֽ���Ȼ�ѧ��Ӧ����ʽ�� ��
��2������5.00 g̼���ƺ�̼�����ƵĻ�����Ӧ��ɺ����������������0.31 g����ԭ�������̼���Ƶ�����Ϊ g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��3��25gпͶ��200mLijŨ�ȵ������У�п������ǡ����ȫ��Ӧ��
��(1)��Ӧ�����ɵ�H2�ڱ�״���µ������
(2)����������HCl�����ʵ���Ũ�ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
���ð������������ᣬ�̶���ȡ����李������������������������Ϊ90%����ת��Ϊ����淋�ת����Ϊ94%����100 t�������������ٶ�����泥�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��2��0 mol��L CuSO4��Һ��1��0 mol��LH2SO4��Һ��100 mL��ϣ������Ϻ���Һ��������ڻ��ǰ������Һ�����֮�ͣ������㣺
��1�����Һ��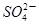 �����ʵ���Ũ��_____________mol��L��
�����ʵ���Ũ��_____________mol��L��
��2������Һ�м����������ۣ����㹻����ʱ���������ʣ�ࡣ��ʱ������������������״����Ϊ_________________L����Һ��Fe2�������ʵ���Ũ����___________mol��L��
��3��������Һ�еμ�1 mol��L��NaOH��Һ��ʹCu2��ǡ����ȫ����������NaOH��Һ�����_____________________mL��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com