
����Ŀ����ɫֲ��걾�ô���ͭ[��CH3COO��2Cu]��������ɫ�����ޡ��ȶ���ij��ѧС���Ʊ�����ͭ���岢�ⶨ��Ʒ��ͭ�ĺ�����ʵ�����¡�
����ͭ������Ʊ�

��1�����У������ӷ���ʽ��ʾ������OH-����Դ��__________��
��2�����У���ѧ����ʽ��__________��
��3�����в��õ�ʵ�鷽�����ݴ���ͭ��������_________��
�ⶨ��Ʒ��ͭ�ĺ���
����ȡa g����ͭ��Ʒ�ھ�����ƿ�У���ϡ�����ܽ⣬�������KI��Һ������CuI��������Һ���ػ�ɫ��
������b molL-1 Na2S2O3����Һ�ζ����е���Һ��dz��ɫʱ�����뼸�ε�����Һ����Һ������������Na2S2O3����Һ�ζ�����ɫ������ʧ��
������������Һ�м���KSCN��Һ�����ҡ������Һ��ɫ���
����������Na2S2O3 ����Һ�ζ�������Һ���յ㣬���ı���Һv mL��
��֪����![]() ��Na2S2O3��Һ��Na2S4O6��Һ��ɫ��Ϊ��ɫ��
��Na2S2O3��Һ��Na2S4O6��Һ��ɫ��Ϊ��ɫ��
��CuI������I2��CuSCN������ˮ�Ҳ�����I2����������I2���������ɫ��
��4�����з�����Ӧ�����ӷ���ʽ��__________��
��5��������ӷ���ʽ˵�������м���KSCN��Ŀ����__________��
��6������ͭ��Ʒ��ͭԪ�ص�����������__________��
���𰸡�CO32-��H2O![]() OH-+HCO3- Cu2��OH��2CO3��4CH3COOH =2 ��CH3COO��2 Cu ��3H2O��CO2�� ����ͭ���ܽ�����¶ȱ仯�ϴ��¶�Խ���ܽ��Խ���¶Ƚ����ܽ�ȼ�С 2Cu2+��4I- = 2CuI����I2 ��ΪCuSCN������I2��ͨ����ӦCuI��s�� ��SCN-
OH-+HCO3- Cu2��OH��2CO3��4CH3COOH =2 ��CH3COO��2 Cu ��3H2O��CO2�� ����ͭ���ܽ�����¶ȱ仯�ϴ��¶�Խ���ܽ��Խ���¶Ƚ����ܽ�ȼ�С 2Cu2+��4I- = 2CuI����I2 ��ΪCuSCN������I2��ͨ����ӦCuI��s�� ��SCN- ![]() CuSCN��s�� ��Cl-��ʹCuI������I2�ͷų�����Na2S2O3��Ӧ�� 6.4bv/a %
CuSCN��s�� ��Cl-��ʹCuI������I2�ͷų�����Na2S2O3��Ӧ�� 6.4bv/a %
��������
ͨ������ͭ��̼���Ʒ�Ӧ�Ƶü�ʽ̼��ͭ����ʽ̼��ͭ������ᷴӦ�õ�����ͭ��Һ����������Ũ������ȴ�ᾧ�����˵õ�����ͭ���塣ͨ�����ζ����ⶨ��Ʒ��ͭ�ĺ�����
��1��̼�������Ϊ��������ӣ���ˮ��Һ�з���ˮ�ⷴӦCO32-��H2O![]() OH-+HCO3-���ʢ�����OH-���ɡ�
OH-+HCO3-���ʢ�����OH-���ɡ�
��2�����д������ʽ̼��ͭ��Ӧ���ɴ���ͭ��������̼��ˮ����ѧ����ʽ��Cu2��OH��2CO3��4CH3COOH =2��CH3COO��2 Cu��3H2O��CO2����
��3������ͭ���ܽ�����¶ȱ仯�ϴ��¶�Խ���ܽ��Խ���¶Ƚ����ܽ�ȼ�С�����Դ���ͭ����ͨ������Ũ������ȴ�ᾧ�����˵õ���
��4�����д���ͭ��⻯�ط�Ӧ���ɵ⻯ͭ�����ʹ���أ�����ͭ���⻯�غʹ���ض��ǿ������������ӷ���ʽ�п��Բ�д����Ӧ�����ӷ���ʽ��2Cu2+��4I- = 2CuI����I2��
��5��������֪��CuI������I2��CuSCN������ˮ�Ҳ�����I2��֪�����м���KSCNͨ����ӦCuI��s����SCN- ![]() CuSCN��s����Cl-��ʹCuI������I2�ͷų�����Na2S2O3��Ӧ��
CuSCN��s����Cl-��ʹCuI������I2�ͷų�����Na2S2O3��Ӧ��
��6������2Cu2+��4I- = 2CuI����I2��![]() ,��֪2Cu2+~ I2~2 S2O32-��n(S2O32-)=n(Cu2+)=
,��֪2Cu2+~ I2~2 S2O32-��n(S2O32-)=n(Cu2+)=![]() �� ����ͭ��Ʒ��ͭԪ�ص�����������
�� ����ͭ��Ʒ��ͭԪ�ص�����������
![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������(D)�������ϵ�ԭ�ϣ�������A�ϳɵõ���

����˵����ȷ����
A. ���л���A���������ϩ���ͱ�ϩ����ȵõ��ģ���÷�Ӧ�ķ�Ӧ�������ڼӳɷ�Ӧ
B. �л���B������O2����������ȩ�����ܸ�NaHCO3��Һ��Ӧ�ų�CO2����
C. �л���C������ͬ���칹���в������з����廯�������
D. �л���D����������̼ԭ��һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪����C2H6��g��![]() C2H4��g��+H2��g�� H1 ��0��
C2H4��g��+H2��g�� H1 ��0��
��C2H6��g��+![]() =2CO2��g��+3H2O��l�� H 2 ��-1559.8 kJ��mol-1
=2CO2��g��+3H2O��l�� H 2 ��-1559.8 kJ��mol-1
��C2H4��g��+3O2��g��=2CO2��g��+2H2O��l�� H 3��-1411.0 kJ��mol-1
����������ȷ����
A.���»��ѹ������ߢ��������ת����
B.���жϼ����յ��������ڳɼ��ų�������
C.��H 2��H 3�ɼ�������е�H
D.�Ʋ�1 mol C2H2��g����ȫȼ�շų�������С��1411.0 kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���о���Դ���������Ȼ����L�ĺϳɶԿ�����ҩ���з�������Ҫ���壬��ϳ�·����Ҫ��Ϊ�����Σ�
I���ϳ��м���F
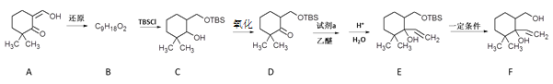
��֪������TBSClΪ![]()
���� 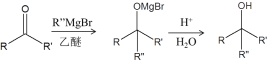
��1��A�������������__________��
��2��B�Ľṹ��ʽ��__________��
��3���Լ�a��__________��
��4��TBSCl��������__________��
II. �ϳ��л���L
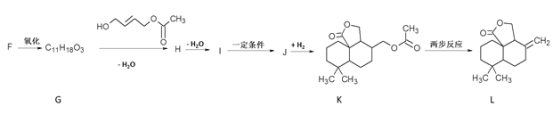
��֪�� ![]()
��5��H�к�������������H�Ľṹ��ʽ��__________��
��6��I��J�ķ�Ӧ����ʽ��__________��
��7��K��L��ת���У�������Ӧ�ķ�Ӧ����������__________��__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������ʵ���Ũ�ȵ����ᡢ����������Һ�ֱ���ڼס������ձ��У���������������������������������Ϊ5:6����ס������ձ��еķ�Ӧ������ֱܷ���
A. �ס����ж��������� B. ���������������м����
C. ��������������������� D. �����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪����ͼ��A�ǽ������������ͼ����ʾ��ת����ϵ���ش��������⣺
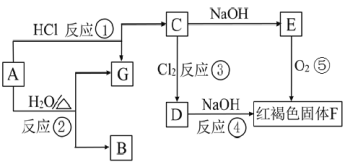
(1)д���������ʶ�Ӧ�Ļ�ѧʽB _______________��E ________________��
(2)д����Ӧ��Ӧ�Ļ�ѧ����ʽ��
��________________________��
��_________________________ ��
��________________________ ��
(3)д����Ӧ����Һ�з�Ӧ�����ӷ���ʽ���� ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)���������ķ�Ӧ����������ͬ����������ͬ�����ﲻͬ����Ӧ��ʵ��Ҳ��ͬ���ݴ˻ش��������⣺
�ٳ����£��ڿ������п������ƣ��ƵĶ���������ɫ�䰵��ʧȥ�����������û�ѧ����ʽ�����������������ԭ��__________________��
�����ڿ�����������������Ӧ�Ļ�ѧ����ʽ��__________________��
�۽�4.6����Ͷ������ˮ�У��������������������__________��
(2)����θҺ����θ��(0.2%��0.4%������)����ɱ�����������������ã���θ��������ܹ������٣������������һ����Χ�ڣ���θ�����ʱ��ҽ��ͨ���á�С�մ�Ƭ����θ��ƽ�����������ơ�
����С�մ�Ƭ(NaHCO3)����θ���������ӷ���ʽΪ____________��
���������ͬʱ����θ����ʱ��÷���θ��ƽ[��Ҫ�ɷ���Al(OH)3]����Ӧ�����ӷ���ʽΪ___________________________________��
��ʵ�����Ʊ�Al(OH)3�ij��÷�������Al2(SO4)3��Һ����εμӰ�ˮ����������д����Ӧ�Ļ�ѧ����ʽ��___________________________________��
(3)�����������ʹ�õĽ���֮һ�����������仯�����֪ʶ������������⡣
���й��Ŵ��Ĵ���֮һ��ָ����������Ȼ��ʯ�Ƴɵģ�����Ҫ�ɷ���_______��
��д����ʯ����Ҫ�ɷֺ����ᷴӦ�����ӷ���ʽ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������㷺Ӧ��������������졢��������
(1)����������ѭ���ֽ�ˮ��H2
��֪��H2O(l)===H2(g)��![]() O2(g)����H1����285.5 kJ/mol
O2(g)����H1����285.5 kJ/mol
6FeO(s)��O2(g) ===2Fe3O4(s)����H2����313.2 kJ/mol
��3FeO(s)��H2O(l)===H2(g)��Fe3O4(s)����H3��___________
(2)Fe2O3��CH4��Ӧ���Ʊ������������������䷴ӦΪ�� 3CH4(g) �� Fe2O3(s) ![]() 2Fe(s) ��6H2(g) ��3CO(g) ��H4
2Fe(s) ��6H2(g) ��3CO(g) ��H4
�ٴ˷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ_________________________________��
�����ݻ���ΪVL������������������ͬ�ܱ������м�����������������������Ȼ��ֱ����amolCO��2a molH2�����������ķ�Ӧ�¶ȷֱ𱣳�T1��T2��T3��������������ͬ������£�ʵ���÷�Ӧ�����е�tminʱCO�����������ͼ1��ʾ����ʱI��II��III����������һ�����ڻ�ѧƽ��״̬����___________(ѡ�����������������)���Ʊ����������������ķ�Ӧ����H4 _____ 0(���������)��
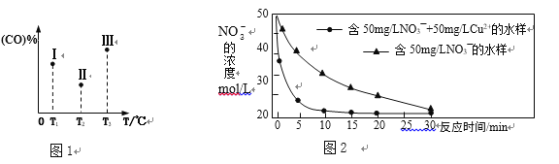
����T���£���ij�����ܱ������м���3molCH4(g)��2mol Fe2O3(s)����������Ӧ����Ӧ��ʼʱѹǿΪP0����Ӧ������10minʱ�ﵽƽ��״̬����ô�ʱ����������ѹǿ����ʼѹǿ��2����10 min����Fe2O3(s)��ʾ��ƽ����Ӧ����Ϊ_______g��min��1�� T���¸÷�Ӧ��Kp = _____________________��T��������ʼʱ��������м���2molCH4(g)��4mol Fe2O3(s)��1molFe(s)��2mol H2(g)��2molCO(g)������ʼʱv (��)______v (��) (������������������������)��
(3)����������ˮ��NO3����Ӧ�����ӷ���ʽΪ 4Fe+ NO3��+10H+=4Fe2++NH4++3H2O
���о����֣���pHƫ�ͽ��ᵼ��NO3����ȥ�����½�����ԭ����_________________��
����ͬ�����£���������ȥ����ͬˮ����NO3���������нϴ���죬ͼ2���������IJ���Ŀ���ԭ����__________________________________________________(��һ��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)��������ָ������ij�¶��£��ɴ��ڱ�״̬�ĸ���Ԫ�ص����ȶ��ĵ������ɱ�״̬�� 1mol ij�����ʵ���ЧӦ����λ���� kJ/mol��ʾ����֪�� 25��������£�
��Ag2O(s)+2HCl(g)�T2AgCl(s)+H2O(l)��H=-324.4 kJ/mol
��2Ag(s)+ ![]() O2(g)�TAg2O(s)��H=-30.56kJ/mol
O2(g)�TAg2O(s)��H=-30.56kJ/mol
�� ![]() H2(g)+
H2(g)+ ![]() Cl2(g)�THCl(g)��H=-92.21 kJ/mol
Cl2(g)�THCl(g)��H=-92.21 kJ/mol
��H2(g)+ ![]() O2(g)�TH2O(l)��H=-285.6 kJ/mol
O2(g)�TH2O(l)��H=-285.6 kJ/mol
��25��ʱ�Ȼ����ı�������Ϊ________ kJ/mol��
(2)ʵ���� 64g �״�[CH3OH(l)]�������г��ȼ������ CO2 �����Һ̬ˮʱ�ų� 1452.8kJ �����������ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽ_________________��
(3)�Լ��顢����Ϊԭ�ϣ�KOH Ϊ����ʣ�����ȼ�ϵ�أ�д���为���ĵ缫��Ӧʽ��________��
(4)��ⷨ��ȡ�й㷺��;�� Na2FeO4������ԭ������ͼ��ʾ��
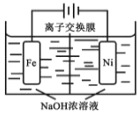
��֪��Na2FeO4 ֻ��ǿ�����������ȶ���
��Na2FeO4�ܹ���ˮ����Ҫԭ����_______________ ��
�������缫��Ӧʽ _______________��
��Ϊʹ����ܽϳ־ý��У�Ӧѡ��_______________ ���ӽ���Ĥ(����������������)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com