��2011?��һģ����ҵ�ϳɰ����Ʊ�����һ��������������������£�
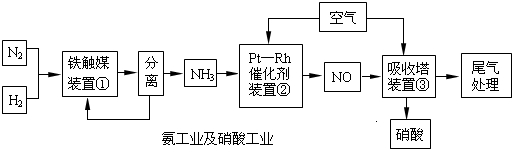
����������⣺
�ϳɰ�
��1��д��װ�â��з�����Ӧ�Ļ�ѧ����ʽ��
��
��2����֪��һ�����¶��½���װ�âٵĵ�������������Ӻϳ��������Ļ������ѹǿ֮��Ϊ5��4����ת����Ϊ
40%
40%
��
���ĽӴ�����ԭ��
��3����900��װ�â��з�Ӧ�У�?
4NH
3��g��+5O
2��g��?4NO��g��+6H
2O��g������H=-905.5kJ?mol
-1 K
1=1��10
53 ��900�棩
4NH
3��g��+4O
2��g��?4N
2O��g��+6H
2O��g������H=-1103kJ?mol
-1 K
2=1��10
61 ��900�棩
4NH
3��g��+3O
2��g��?2N
2��g��+6H
2O��g������H=-1267kJ?mol
-1 K
3=1��10
67 ��900�棩
�������з�Ӧ�⣬����һ����������ã�
4NH
3��g��+6NO��g��?5N
2��g��+6H
2O��g������H=-1804kJ?mol
-1�������ܷ�������һ�������ķֽ⣮
����Ȼ�ѧ����ʽ��2NO��g��?N
2��g��+O
2��g������H=
-180.75 kJ?mol-1
-180.75 kJ?mol-1
��
��4����-��Ͻ�����Ĵ�����Ϊ���ͽ�������̣���ͼ��ʾ��
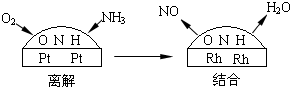
���ڲ���NO��ˮ���ӵ���������С�������ڵ�����ԭ�ӽ�ϣ�ʹ��NO��ˮ�����ڲ������Ѹ������������У���û��ʹ�ò�-��Ͻ�������������������Ҫ����
������ˮ����
������ˮ����
��˵�������Է�Ӧ��
ѡ����
ѡ����
��
��5���¶ȶ�һ���������ʵ�Ӱ��
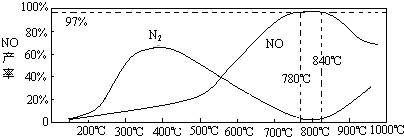
���¶ȴ���900��ʱ��NO�IJ����½���ԭ��
A��B
A��B
��ѡ����ţ�
A���ٽ���һ�������ķֽ�
B���ٽ��˰��ķֽ�
C��ʹ����һ�������ķ�Ӧƽ���ƶ������ɸ���N
2��6�����Ṥҵ��β������Na
2CO
3��Һ������β����NO��NO
2��ȫ�������գ�д����Na
2CO
3��Һ���յķ�Ӧ����ʽ
NO+NO2+Na2CO3�T2NaNO2+CO2��2NO2+Na2CO3�TNaNO2+NaNO3+CO2
NO+NO2+Na2CO3�T2NaNO2+CO2��2NO2+Na2CO3�TNaNO2+NaNO3+CO2
��






 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�