
��ҵ�ϳɰ��ķ�ӦN2��3H2�� 2NH3�������仯����ͼ��ʾ����ش��й����⣺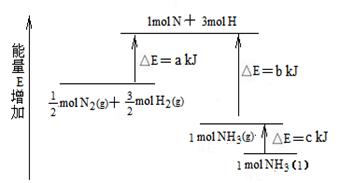
��1�����ϳ� 1 mol NH3(l) ____________������ա������ų�����_____________kJ��������
��2������֪���� lmol H��H ����lmol N��H ����lmol N��N ���ֱ���Ҫ��������436kJ��391kJ��946kJ������ͼ�еģ_______________kJ��1 mol N2(g) ��ȫ��Ӧ����NH3(g)�����������仯Ϊ ______KJ��
��3�����ƲⷴӦ 2NH3(l)�� N2(g)��3H2(g) �ȷ�Ӧ 2NH3 (g)�� N2(g)��3H2(g)______________������ա������ų�����������_____________����ࡱ�����١�����
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| T/K | 303 | 313 | 323 | 353 |
| NH3������/��10-6mol�� | 4.8 | 5.9 | 6.0 | 2.0 |
| 3 |
| 2 |
 2NH3��g���������ݻ�Ϊ2.0L���ܱ������г���0.60mol N2��g����1.60molH2��g������Ӧ��һ�������´ﵽƽ��ʱ��NH3�����ʵ���������NH3�����ʵ����뷴Ӧ��ϵ���ܵ����ʵ���֮�ȣ�Ϊ
2NH3��g���������ݻ�Ϊ2.0L���ܱ������г���0.60mol N2��g����1.60molH2��g������Ӧ��һ�������´ﵽƽ��ʱ��NH3�����ʵ���������NH3�����ʵ����뷴Ӧ��ϵ���ܵ����ʵ���֮�ȣ�Ϊ| 4 |
| 7 |
 N2��g��+3H2��g����ƽ�ⳣ����
N2��g��+3H2��g����ƽ�ⳣ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ���һ��2010��2011ѧ���һ��һ�ν��Կ��Ի�ѧ���� ���ͣ�022
��ҵ�ϳɰ��ķ�ӦN2��3H2��2NH3�������仯����ͼ��ʾ����ش��й����⣺
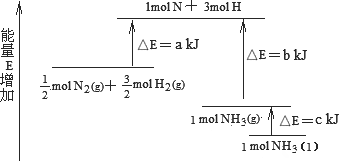
(1)�ϳ�1 mol��NH3(l)________(����ա������ų���)________kJ��������
(2)��֪��
��1 mol��H��H����1 mol��N��H����1 mol��N��N ���ֱ���Ҫ��������436 kJ��391 kJ��946 kJ������ͼ�е�a��________kJ��1 mol��N2(g)��ȫ��Ӧ����NH3(g)�����������仯Ϊ________KJ��(3)�ƲⷴӦ2NH3(l)��N2(g)��3H2(g)�ȷ�Ӧ2NH3(g)��N2(g)��3H2(g)________(����ա������ų���)������________(��ࡱ�����١�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ѧϰ�ܱ�����ѧ���˽̿α�߶���(ѡ��4)��2009��2010ѧ�ꡡ��7�ڡ��ܵ�163�� �˽̿α��(ѡ��4) ���ͣ�013
|
�ڹ�ҵ�ϳɰ��ķ�Ӧ N2��3H2 | |
| [����] | |
A�� |
ʹƽ��������Ӧ�����ƶ� |
B�� |
û�д����÷�Ӧ���ܷ��� |
C�� |
ʹ��ѧ��Ӧ�������� |
D�� |
�����淴Ӧ�ķ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com