
| | | A��B | A=B | A��B |
| CO | ���ܣ�kJ/mol�� | 357.7 | 798.9 | 1071.9 |
| ���ܲ�ֵkJ/mol�� | 441.2 273 | |||
| N2 | ���ܣ�kJ/mol�� | 154.8 | 418.4 | 941.7 |
| ���ܲ�ֵkJ/mol�� | 263.6 523.3 | |||


 A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д� ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Rԭ�ӵĵ��Ӳ�����N�ĵ��Ӳ�����2 |
| B��RԪ�ص���������ϼ���NO3���е�N�Ļ��ϼ���� |
| C��R������������N�ĵ�������10 |
| D��R��NΪͬ��Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
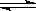 SO3��g������H����98 kJ��mol��1����ʼʱ��100 L���ܱ������м���4.0 mol SO2(g)��10.0 mol O2(g)������Ӧ�ﵽƽ��ʱ���ų�����196 kJ�����¶���ƽ�ⳣ��K�� �������£������������ټ���4mol SO2(g)�������´ﵽƽ��ʱSO2����ת���� ԭƽ��ʱSO2ת���ʣ�ѡ���������������������
SO3��g������H����98 kJ��mol��1����ʼʱ��100 L���ܱ������м���4.0 mol SO2(g)��10.0 mol O2(g)������Ӧ�ﵽƽ��ʱ���ų�����196 kJ�����¶���ƽ�ⳣ��K�� �������£������������ټ���4mol SO2(g)�������´ﵽƽ��ʱSO2����ת���� ԭƽ��ʱSO2ת���ʣ�ѡ����������������������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����n = 2������ӵ�����ṹΪV�� |
| B����n = 3������ӽṹΪ������ |
| C����n = 4������ӵ�����ṹΪ���������� |
| D������˵��������ȷ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ж���
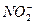 ��BF3�ֱ�Ϊֱ���Ρ�V�κ�ƽ�������Ρ����õȵ���ԭ��˵�����з��ӻ����ӵijɼ�����ͼ��ι��͡�
��BF3�ֱ�Ϊֱ���Ρ�V�κ�ƽ�������Ρ����õȵ���ԭ��˵�����з��ӻ����ӵijɼ�����ͼ��ι��͡�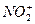 ��
��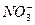 ��
��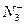 ��
��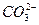
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ϡ������ľ����в����ڷ��Ӽ������� |
B��C��12��ԭ����ɷ���Ϊ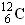 ��������̼���ӵĽṹʽΪO=C=O ��������̼���ӵĽṹʽΪO=C=O |
| C��Na2O��Na2O2������ѧ��������ȫ��ͬ |
| D��Br2������ľ̿����ʱ���ۼ����ƻ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��


| A��BaSO4���ܽ�ȡ�Ksp������ | B��BaSO4���ܽ������Ksp���� | C��BaSO4���ܽ�Ȳ��䣬Ksp���� | D��BaSO4���ܽ�ȡ�Ksp������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ȵ⻯������ʹ֮�ֽ�ֻ��˷����Ӽ������� |
| B���Ȼ�������ˮ�ܵ����H����Cl��,�����Ȼ��������ӻ����� |
| C������������ת�����ڻ�ѧ�仯 |
| D���⾧��͵�������ͬ�������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com