



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
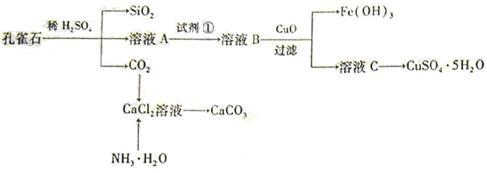
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
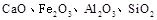 ×é³É£¬ÓÉĮńŹÆæóŹÆÖĘČ”ĀČ»ÆøĘŗĶŃõ»ÆĀĮµÄŹµŃé²½ÖčČēĻĀ£ŗ
×é³É£¬ÓÉĮńŹÆæóŹÆÖĘČ”ĀČ»ÆøĘŗĶŃõ»ÆĀĮµÄŹµŃé²½ÖčČēĻĀ£ŗ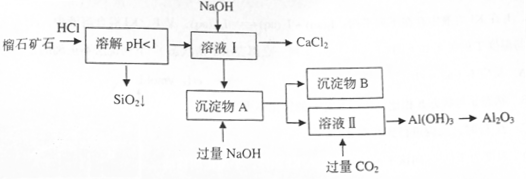
 Ķā£¬»¹ŗ¬ÓŠµÄ½šŹōĄė×ÓŹĒ ”£
Ķā£¬»¹ŗ¬ÓŠµÄ½šŹōĄė×ÓŹĒ ”£
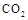 ĘųĢå²¢ĶØČėČÜŅŗIIÖŠ£¬½į¹ūƻӊ³Įµķ²śÉś£¬æÉÄܵÄŌŹĒ £»ĪŖĮĖÄܲśÉś³ĮµķøĆĶ¬Ń§¶ŌĶ¼I×°ÖĆ½ųŠŠĮĖøĽų£¬øĽųµÄ·½·ØĪŖ ”£
ĘųĢå²¢ĶØČėČÜŅŗIIÖŠ£¬½į¹ūƻӊ³Įµķ²śÉś£¬æÉÄܵÄŌŹĒ £»ĪŖĮĖÄܲśÉś³ĮµķøĆĶ¬Ń§¶ŌĶ¼I×°ÖĆ½ųŠŠĮĖøĽų£¬øĽųµÄ·½·ØĪŖ ”£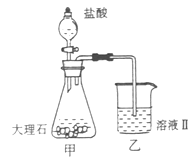
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
 2H++ Fe£ØOH£©2ŌŚ25”ꏱµÄĘ½ŗā³£Źż
2H++ Fe£ØOH£©2ŌŚ25”ꏱµÄĘ½ŗā³£Źż ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
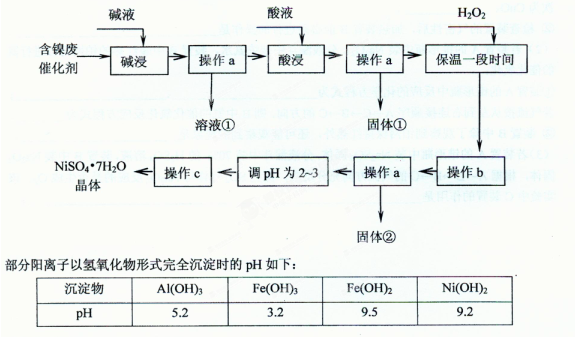
| ĪĀ¶Č | 10”ę | 20”ę | 30”ę | Čܽā¶Č£ŗ20”ęNaF”Ŗ4 0”ęNH4F”Ŗ100£» ³£ĪĀNa2SiF6Ī¢ČÜÓŚĖ® |
| NH4ClČܽā¶Č | 33 .3 .3 | 37.2 | 41.4 |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
 £©£¬¼ģŃéČÜŅŗAÖŠFe3+µÄ×ī¼ŃŹŌ¼ĮĪŖ””””””””£ØĢī“śŗÅ£©”£
£©£¬¼ģŃéČÜŅŗAÖŠFe3+µÄ×ī¼ŃŹŌ¼ĮĪŖ””””””””£ØĢī“śŗÅ£©”£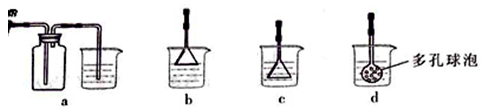
 £©Óū²ā¶ØČÜŅŗAÖŠFe2+µÄÅØ¶Č£¬ŠčŅŖÓĆČŻĮæĘæÅäÖĘij±ź×¼ČÜŅŗ£¬¶ØČŻŹ±ø©ŹÓČŻĮæĘæµÄæĢ¶ČĻߣ¬»įŹ¹ÅäÖʵÄÅØ¶Č ”££ØĢī”°Ę«øß”±”¢”°Ę«µĶ”±”¢”°ĪŽÓ°Ļģ”±”££©
£©Óū²ā¶ØČÜŅŗAÖŠFe2+µÄÅØ¶Č£¬ŠčŅŖÓĆČŻĮæĘæÅäÖĘij±ź×¼ČÜŅŗ£¬¶ØČŻŹ±ø©ŹÓČŻĮæĘæµÄæĢ¶ČĻߣ¬»įŹ¹ÅäÖʵÄÅØ¶Č ”££ØĢī”°Ę«øß”±”¢”°Ę«µĶ”±”¢”°ĪŽÓ°Ļģ”±”££©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
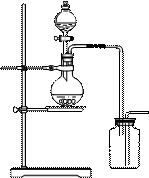
 »įĆ÷ĻŌŌö¼Ó”£
»įĆ÷ĻŌŌö¼Ó”£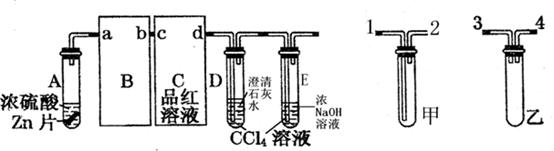
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
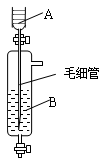
| A£®A֊װÅØĮņĖį£¬B֊װÅØŃĪĖį |
| B£®A֊װÅØŃĪĖį£¬B֊װÅØĮņĖį |
| C£®A֊װĒāŃõ»ÆÄĘÅØČÜŅŗ£¬B֊װÅØ°±Ė® |
| D£®A֊װÅØ°±Ė®£¬B֊װĒāŃõ»ÆÄĘÅØČÜŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
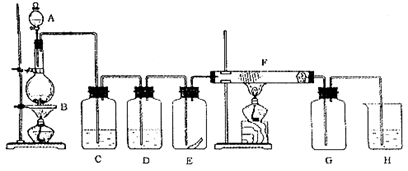
 ŠŠĻą¹ŲŠŌÖŹŹµŃ锣£ØŹµŃé×°ÖĆČēÓŅĶ¼ĖłŹ¾£©
ŠŠĻą¹ŲŠŌÖŹŹµŃ锣£ØŹµŃé×°ÖĆČēÓŅĶ¼ĖłŹ¾£©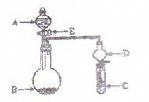
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com