
| A�� | ���Ӵ�������H2SO4ʱ���������εķ�Ӧԭ��Ϊ��2SO2��g��+O2��g��$?_{��}^{����}$2SO3��g����H��0 | |
| B�� | ��ˮ��þ����Ҫ����Ϊ����ˮ$\stackrel{CaCO_{3}��s��}{��}$Mg��OH��2��s��$\stackrel{����}{��}$MgCl2��aq��$\stackrel{���}{��}$Mg��l��+Cl2��g�� | |
| C�� | ��ͨˮ�����Ҫ�ɷ��ǹ���� | |
| D�� | �������Ҫ�ɷ��������������� |
���� A��������Ϊ���淴Ӧ���ҷ��ȣ�
B��̼������յõ�CaO��CaO�뺣ˮ��þ���ӷ�Ӧ����Mg��OH��2���ҵ������MgCl2ұ��Mg��
C����ͨˮ�����Ҫ�ɷ��ǹ������ơ�������ơ��������ƣ�
D���������Ҫ�ɷ���Al2O3•2SiO2•2H2O��
��� �⣺A�����Ӵ�������H2SO4ʱ�����������Ƕ���������������Ϊ���������䷴Ӧ����Ϊ2SO2��g��+O2��g��$?_{��}^{����}$2SO3��g����H��0����A��ȷ��
B����ˮ��þ�Ĺ����У���ˮ��Ӧ����ʯ���������̼��ƣ�����Ȼ�þ��Һ�õ�����������������þ���ò���Mg���ʣ�����Ҫ��õ���þʱӦ������ڵ�MgCl2����B����
C����ͨˮ�����Ҫ�ɷ��ǹ������ơ�������ơ��������ƣ���C����
D���������Ҫ�ɷ���Al2O3•2SiO2•2H2O��������������������D����
��ѡA��
���� ���⿼�麣����ԴӦ�ü����ʵ����ʣ�Ϊ��Ƶ���㣬�������ʵ����ʡ������ķ�Ӧ����������;Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬ע��Ԫ�ػ�����֪ʶ�뷴Ӧԭ����Ӧ�ã���Ŀ�ѶȲ���


 �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д� ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д� ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д� ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

 $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$ +CO2��
+CO2�� $��_{��}^{NaOH��aq��}$
$��_{��}^{NaOH��aq��}$ +H2O
+H2O $��_{����H+}^{����_{10}%NaOH��Cu}$
$��_{����H+}^{����_{10}%NaOH��Cu}$
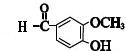 +CH3COCH2COCH3$��_{��}^{NaOH��aq��}$
+CH3COCH2COCH3$��_{��}^{NaOH��aq��}$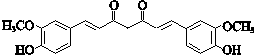 +2H2O��
+2H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ����̼���ƺ�̼������ | |
| B�� |  �Ƶ�ȼ�� | |
| C�� | 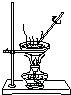 ֱ�������Ȼ�����Һ����Ȼ������� | |
| D�� | 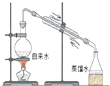 ʵ������ȡ����ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
��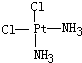 ��
��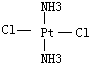 ��
��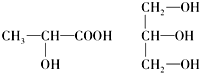
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
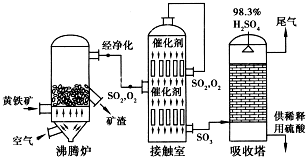
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.5molO3��11.2LO2�����ķ�����һ����� | |
| B�� | 25����60��ʱ��ˮ��pH��� | |
| C�� | �к͵�����������ʵ�����Ũ�ȵ�����ʹ��������ĵ�n��NaOH����� | |
| D�� | 2SO2��g��+O2��g��=2SO3��g����4SO2��g��+2O2��g��=4SO3��g���ġ�H��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
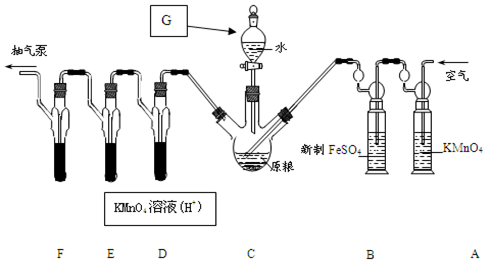
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com