
�����ʽṹ�����ʡ���ԭ�ӡ�����ˮƽ�ϰ���������ʶ���ʹ��ɵĹ��ɣ�����֮�䲻ͬ��������Ϊ�������о���ͬ�������ʵ��й����ʣ������ʽṹ�������ʵ��ӽ�Ԥ�����ʵ��й����ʡ�
(1)����˵����ȷ����________(�����)��
A��Ԫ�ص縺���ɴ�С��˳��Ϊ��F��O��N
B��һ�������Ӻ�3���м���6���Ҽ�
C���Ȼ��ƺ��Ȼ�菉����������ӵ���λ����ͬ
D����һ�����ܵĴ�СΪ��Br��Se��As

(2)���ݵȵ�����ԭ�����ʻ���(OCS)���ӵĽṹʽӦΪ________������(COCl2)�����ڸ�ԭ������㶼����8�����ȶ��ṹ����������ӵ����幹��Ϊ________(����������)��
(3)Cu2����̬�ĵ����Ų�ʽΪ____________________________________��
������ͭ��Һ�м��������ˮ��Ȼ����������Ҵ�����Һ�л���������ɫ��[Cu(NH3)4]SO4���壬����������λԭ�ӵ��ӻ���ʽΪ________��
���� ��(1)ͬһ������ԭ�������ĵ������縺����������A����ȷ���������в�����C��C����ֻ����һ����м���B������Ȼ����������ӵ���λ��Ϊ 6 ���Ȼ���������ӵ���λ��Ϊ8��C�����As���ڢ�A �壬�������������Ӧ��Ϊ��Br��As��Se ��D�����(2)�ʻ�����CO2�ǵȵ����壬�ṹʽ������CO2�����ݸ�ԭ�ӵijɼ��ص㣬��֪�����д���C��O����̼ԭ�Ӳ�ȡ sp2�ӻ������幹��Ϊƽ�������Ρ�(3)Cu2���ĵ����Ų�ʽΪ[Ar]3d9��[Cu(NH3)4]2������λ��ԭ���γ�4���Ҽ�����ȡ sp3 �ӻ���
�� ��(1)A��(2)O��C��S��ƽ��������
(3)1s22s22p63s23p63d9 (��[Ar]3d9)��sp3�ӻ�


 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ������Ԥ����ȷ���ǣ�������
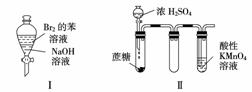 |
A��ʵ������ã��ϲ���Һ��ɫ���ֲ���
B��ʵ�������KMnO4��Һ�г������ݣ�����ɫ����ȥ
C��ʵ�����ϡHNO3Ƭ�̣���Һ�������ݲ��������ƿ��ʼ�ձ�����ɫ
D��ʵ��������������Һ�����ɫ��ֹͣ���ȣ�������ͨ����ϵʱ�����������ЧӦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͨ����ʳ���Ϳɻ��ij�����л�������X������Է�������Ϊ46������̼����������Ϊ52.2%�������������Ϊ13.0%��
(1)X�ķ���ʽ��________________________________________________________________________��
(2)X������Ʒ�Ӧ�ų���������Ӧ�Ļ�ѧ����ʽ��__________________________(�л����ýṹ��ʽ����)��
(3)X������е�������ͭ�����������·�Ӧ����Y����Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
(4)X��������������Һ��Ӧ������Z��Z��������______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ԭ��Ӧ2H2��O2===2H2O��Ƴ�ԭ��ء�
(1)��������������������������Һ����ȼ�ϵ�أ���ͨ�������Ӧ��________������ͨ���������________���缫��ӦΪ������__________________������______________________��
(2)����KOH��Һ��Ϊϡ�������������Һ����缫��ӦΪ������________������________________��
(3)����H2��ΪCH3OH��KOH��Һ���������Һ����缫��ӦΪ������_____________________________________________��
����__________����ط�ӦΪ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������NH3��BF3����ͨ����λ���γ�NH3��BF3������˵����ȷ����(����)��
A��NH3��BF3����������
B��NH3��BF3���Ǽ��Է���
C��NH3��BF3�и�ԭ�Ӷ��ﵽ8�����ȶ��ṹ
D��NH3��BF3�У�NH3�ṩ�µ��Ӷԣ�BF3�ṩ�չ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и������������ֲ���6���� (����)��
A����NaCl�����У���һ��Na��������Ҿ�����ȵ�Cl���ĸ���
B���ڽ��ʯ�����У���С�Ļ��ϵ�̼ԭ�Ӹ���
C���ڶ������辧���У���С�Ļ��ϵ�ԭ�Ӹ���
D����ʯī�����Ƭ��ṹ�У���С�Ļ��ϵ�̼ԭ�Ӹ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼΪCaF2��H3BO3(��״�ṹ�����ڵ�H3BO3����ͨ��������)������ͭ���־���Ľṹʾ��ͼ����ش��������⣺
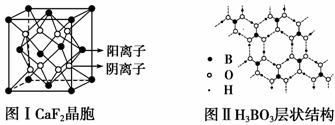
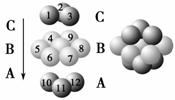
ͼ��ͭ������ͭԭ�Ӷѻ�ģ��
(1)ͼ����ʾ��CaF2��������Ca2������ҵȾ����F����Ϊ________________��ͼ����δ��ŵ�ͭԭ���γɾ������Χ����ڵ�ͭԭ����Ϊ___________________________________________��
(2)ͼ����ʾ�����ʽṹ�������ܲ��Ѵ�8���ӽṹ��ԭ����________��H3BO3 ������Bԭ�Ӹ����뼫�Լ�������Ϊ____________��
(3)����ͭ���кܺõ���չ�ԡ������ԡ������ԣ��Դ�������Ľ�������________���ۡ�
(4)���־������۵���͵���________(�ѧʽ)���侧�������ۻ�ʱ���˷�����֮��������Ϊ_________________��
(5)��֪�������������Ca2���˼����Ϊa��10��8cm�����CaF2����ľ���ʾ��ͼ��CaF2������ܶ�Ϊ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������Ϊ0.052(5.2%)��NaOH��Һ1 L(�ܶ�Ϊ1.06 g��cm3)�ò��缫��⣬����Һ��NaOH�����������ı���0.010(1.0%)ʱֹͣ��⣬���ʱ��Һ��Ӧ���ϵĹ�ϵ��(����)
| NaOH���������� | ���������������/g | ���������������/g | |
| A | 6.2% | 19 | 152 |
| B | 6.2% | 152 | 19 |
| C | 4.2% | 1.2 | 9.4 |
| D | 4.2% | 9.4 | 1.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y����Ԫ�ص�ԭ�ӵ�������֮��Ϊ20����Ԫ���γɵĻ�������ˮ��Һ���ܵ�������Ӳ�ṹ��ͬ�����������ӣ���X��Y�γɵĻ�������(����)
A��MgF2 B��NaF
C��LiCl D��Na2O
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com