
��15�֣����ڼס��ҡ�����������������Һ��������Ϣ��
�ٷֱ�NH4+��Na+��Al3+��Ba2+��Ag+��NO3-��Cl-��SO42-��Br-��CO32-�����еĸ�һ�����(���ظ�)��
�����мס�������������Һ�����ԣ�����Һ�ʼ��ԣ�
�ۼס��ҷ�Ӧ���ɰ�ɫ���������壬���ɷֱ���ס��ҡ�����Ӧ���ɰ�ɫ������
��ش��������⣺
���û�ѧʽ��ʾ�������ʣ��� ���� ��
���������ӷ���ʽ��ʾ����Һ�����Ե�ԭ�� ��
���������ӷ���ʽ��ʾ�����ҵķ�Ӧ�� ��
�ȼ������Һ�м������ӵķ������ȼ� �Լ����ټ� �Լ����۲쵽 ����֤���������Ӵ��ڡ�
�����������γɵİ�ɫ�������ܶȻ�����Ksp=1.8��10-20����1 L 1mol/L�ı���Һ��1 L 1 mol/L�Ķ���Һ��ϳ�ַ�Ӧ��������Һ���ʱ��С����仯���γɳ����������ӵ�Ũ��ԼΪ mol/L��
��Na2CO3��2�֣���BaCl2��2�֣�
��NH4����H2O NH3��H2O��H�� ��2�֣�
NH3��H2O��H�� ��2�֣�
��2Al3++3CO32-+3H2O=2Al(OH)3��+3CO2����2�֣�
�����������ᣨ1�֣���BaCl2��1�֣�����ɫ������1�֣���3.6��10-20 mol/L��4�֣�
���������ס��ҷ�Ӧ���ɰ�ɫ���������壬������Һ�ʼ��ԣ�����Һ�����ԣ�������к���CO32-�����к���Al3+�����߷�Ӧ��������������CO2����������̼���ơ�������ֻ����NO3����ϣ���һ�������������������ɷֱ���ס��ҡ�����Ӧ���ɰ�ɫ�������Ҷ�����Һ�����Եģ����Զ����Ȼ��������������������������������������廯李�
��2��NH4��ˮ�������ԣ�����ʽΪNH4����H2O NH3��H2O��H����
NH3��H2O��H����
��3��������ˮ�������ԣ�CO32��ˮ���Լ��ԣ�������ٽ�������ʽΪ2Al3++3CO32-+3H2O=2Al(OH)3��+3CO2����
��4������SO42��Ҫ�ų����������ӵĸ��ţ�����ҪҪ�������ᣬ�ټ����Ȼ�����Һ�����������ɫ��������֤��SO42�����Ӵ��ڡ�
��5���������ⷴӦ����Һ��������Ũ����0.5mol/L�����Ը����ܶȻ���������ʽ��֪����Һ�������ӵ��� 3.6��10-20 mol/L��
3.6��10-20 mol/L��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
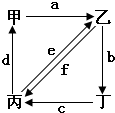 ��ͼ��ʾ���ס��ҡ��������ֱ����CO2��Na2CO3��NaOH��NaHCO3�������ʣ�a��b��c��d��e��f�ֱ��ʾ�������ʼ��ת����ϵ������ͼ���������ʼ��ת����ͨ��һ����Ӧ����ʵ�ֵ��� ��������
��ͼ��ʾ���ס��ҡ��������ֱ����CO2��Na2CO3��NaOH��NaHCO3�������ʣ�a��b��c��d��e��f�ֱ��ʾ�������ʼ��ת����ϵ������ͼ���������ʼ��ת����ͨ��һ����Ӧ����ʵ�ֵ��� ���������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ������ѧ�����ڶ��ζο������ۺϻ�ѧ�Ծ����������� ���ͣ������
(16��)���ڼס��ҡ�����������������Һ��������Ϣ��
�ٷֱ�NH4+��Na+��Al3+��Ba2+��Ag+��NO3����Cl����SO42-��Br����CO32�������еĸ�һ�����(���ظ�)��
�����мס�������������Һ�����ԣ�����Һ�ʼ��ԡ�
�ۼס��ҷ�Ӧ���ɰ�ɫ���������壬���ɷֱ���ס��ҡ�����Ӧ���ɰ�ɫ������
��ش��������⣺
���û�ѧʽ��ʾ�������ʣ��� ���� ��
���������ӷ���ʽ��ʾ����Һ�����Ե�ԭ�� ��
���������ӷ���ʽ��ʾ�����ҵķ�Ӧ�� ��
�ȼ������Һ�м������ӵķ������ȼ� �Լ����ټ� �Լ����۲쵽
����֤���������Ӵ��ڡ�
�����������γɵİ�ɫ�������ܶȻ�����Ksp=1.8��10��20����1 L 1mol/L�ı���Һ��1 L 1 mol/L�Ķ���Һ��ϳ�ַ�Ӧ��������Һ���ʱ��С����仯���γɳ����������ӵ�Ũ��ԼΪ mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ӱ�ʡ��У���˸�����ѧ�ڵ��п��������ۺϻ�ѧ�Ծ����������� ���ͣ������
��15�֣����ڼס��ҡ�����������������Һ��������Ϣ��
�ٷֱ�NH4+��Na+��Al3+��Ba2+��Ag+��NO3-��Cl-��SO42-��Br-��CO32-�����еĸ�һ�����(���ظ�)��
�����мס�������������Һ�����ԣ�����Һ�ʼ��ԣ�
�ۼס��ҷ�Ӧ���ɰ�ɫ���������壬���ɷֱ���ס��ҡ�����Ӧ���ɰ�ɫ������
��ش��������⣺
���û�ѧʽ��ʾ�������ʣ��� ���� ��
���������ӷ���ʽ��ʾ����Һ�����Ե�ԭ�� ��
���������ӷ���ʽ��ʾ�����ҵķ�Ӧ�� ��
�ȼ������Һ�м������ӵķ������ȼ� �Լ����ټ� �Լ����۲쵽 ����֤���������Ӵ��ڡ�
�����������γɵİ�ɫ�������ܶȻ�����Ksp=1.8��10-20����1 L 1mol/L�ı���Һ��1 L 1 mol/L�Ķ���Һ��ϳ�ַ�Ӧ��������Һ���ʱ��С����仯���γɳ����������ӵ�Ũ��ԼΪ mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�����ڶ��ζο������ۺϻ�ѧ�Ծ��������棩 ���ͣ������
(16��)���ڼס��ҡ�����������������Һ��������Ϣ��
�ٷֱ�NH4+��Na+��Al3+��Ba2+��Ag+��NO3����Cl����SO42-��Br����CO32�������еĸ�һ�����(���ظ�)��
�����мס�������������Һ�����ԣ�����Һ�ʼ��ԡ�
�ۼס��ҷ�Ӧ���ɰ�ɫ���������壬���ɷֱ���ס��ҡ�����Ӧ���ɰ�ɫ������
��ش��������⣺
���û�ѧʽ��ʾ�������ʣ��� ���� ��
���������ӷ���ʽ��ʾ����Һ�����Ե�ԭ�� ��
���������ӷ���ʽ��ʾ�����ҵķ�Ӧ�� ��
�ȼ������Һ�м������ӵķ������ȼ� �Լ����ټ� �Լ����۲쵽
����֤���������Ӵ��ڡ�
�����������γɵİ�ɫ�������ܶȻ�����Ksp=1.8��10��20����1 L 1mol/L�ı���Һ��1 L 1 mol/L�Ķ���Һ��ϳ�ַ�Ӧ��������Һ���ʱ��С����仯���γɳ����������ӵ�Ũ��ԼΪ mol/L��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com