
 A��B��C��D��E��F��G��H�Ǻ˵������������Ķ���������Ԫ�أ�Ԫ��A��ԭ�Ӱ뾶������Ԫ������С�ģ�A��Eͬ���壬B��C��Dͬ���ڣ�D��G������������ȣ�G��������ΪD��2����Ԫ��B��һ�ֳ������ʿ������Ե缫���ϣ�������������Ϊ�����������壮B��D��G��������֮�͵���F��H��������֮�ͣ�I�������ճ��������������Ľ������ױ���ʴ�����ش��������⣺
A��B��C��D��E��F��G��H�Ǻ˵������������Ķ���������Ԫ�أ�Ԫ��A��ԭ�Ӱ뾶������Ԫ������С�ģ�A��Eͬ���壬B��C��Dͬ���ڣ�D��G������������ȣ�G��������ΪD��2����Ԫ��B��һ�ֳ������ʿ������Ե缫���ϣ�������������Ϊ�����������壮B��D��G��������֮�͵���F��H��������֮�ͣ�I�������ճ��������������Ľ������ױ���ʴ�����ش��������⣺| 0.784L |
| 22.4L/mol |
| 0.784L |
| 22.4L/mol |
| 0.04mol |
| 0.4L |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����NaHSO4��Һ�еμ�Ba��OH��2��SO42-������ȫ��2H++SO42-+Ba2++2OH-=BaSO4��+2H2O |
| B�������ʯ��ˮ�м��������NaHCO3��Һ��Ca2++OH-+HCO3-�TCaCO3��+H2O |
| C��FeBr2��Һ�м����������ˮ 2Fe2++2Br-+2Cl2�TBr2+2Fe3++4Cl- |
| D����NaAlO2��Һ��ͨ������CO2��2AlO2-+CO2+3H2O=2Al��OH��3��+CO32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
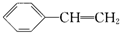 ����������������ǣ�������
����������������ǣ�������| A�����Ժ���ˮ��Ӧ |
| B������ϩ��ͬϵ�� |
| C����ѧʽΪC8H8 |
| D��1 mol����ϩ�����Ժ�4 mol H2�����ӳɷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����pH=1����Һ�У�Fe2+��NO3-��SO42-��Na+ |
| B�����ܽ�Al��OH��3����Һ�У�NH4+��SO42-��Cl-��HCO3- |
| C����̪�ʺ�ɫ����Һ�У�K+��Na+��AlO2-��NO3- |
| D�����д���Fe2+����Һ�У�H+��K+��ClO-��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ÿ�տ�ѧ��վ���±���������÷���������о���Ա�����̲躬������������Ѫ��ϸ���ijɷ�-����ûʳ�Ӷ�����ûʳ����������EGCG�����о���ʾ���óɷ�ͨ������Ѫ��ϸ������������źŴ��ݣ���ʹѪ������Ѫ�����а�ϸ����ɱ����������֪EGCG�Ľṹ��ʽ��ͼ���й�EGCG��˵������ȷ���ǣ�������
����ÿ�տ�ѧ��վ���±���������÷���������о���Ա�����̲躬������������Ѫ��ϸ���ijɷ�-����ûʳ�Ӷ�����ûʳ����������EGCG�����о���ʾ���óɷ�ͨ������Ѫ��ϸ������������źŴ��ݣ���ʹѪ������Ѫ�����а�ϸ����ɱ����������֪EGCG�Ľṹ��ʽ��ͼ���й�EGCG��˵������ȷ���ǣ�������| A��EGCG�����к�����������̼ԭ�� |
| B��EGCG��FeCl3��Һ�ܷ�����ɫ��Ӧ |
| C��EGCG�ڿ����в��ױ����� |
| D��EGCG��������������Һ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��һ������ | �뾶 | �縺�� | �е� |
| N | Al | O | CH4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com