
�������й㷺����;�����к�������Fe��Zn��Cu��Pt�����ʣ����õ�ⷨ�Ʊ��ߴ��ȵ���������������ȷ���ǣ���֪��������Fe2+��Ni2+��Cu2+��( )
A������������ԭ��Ӧ����缫��Ӧʽ��Ni2+ + 2e�� Ni
Ni
B���������У����������ļ����������������������
C��������Һ�д��ڵĽ���������ֻ��Fe2+ ��Zn2+
D�������۵ײ�����������ֻ��Cu��Pt
 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶��ϵ�һ�ο��Ի�ѧ���������棩 ���ͣ�ѡ����
��(N2H4)�ǻ����������ȼ�ϣ�����N2O4��Ӧʱ��N2O4Ϊ�����������ɵ�����ˮ��������֪��
N2(g)��2O2(g)��N2O4(g) ��H����8.7 kJ/mol��
N2H4(g)��O2(g)��N2(g)��2H2O(g) ��H����534.0 kJ/mol��
���б�ʾ�¸�N2O4��Ӧ���Ȼ�ѧ����ʽ����ȷ����( )
A��2N2H4(g)��N2O4(g)��3N2(g) ��4H2O(g) ��H����542.7 kJ/mol
B��2N2H4(g)��N2O4(g)��3N2(g)��4H2O(g) ��H����1059.3 kJ/mol
C��N2H4(g)�� N2O4(g)��
N2O4(g)�� N2(g)��2H2O(g) ��H����1076.7 kJ/mol
N2(g)��2H2O(g) ��H����1076.7 kJ/mol
D��2N2H4(g)��N2O4(g)��3N2(g)��4H2O(g) ��H����1076.7 kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017���㽭ʡ�����и�����ѧ����Ӧ�Կ��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��������Ϊ��������ƿ�����ǣ� ��

A. B. C. D.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ɽ��ʡ�����и�����ѧ��10���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�۲����м���װ��ʾ��ͼ���й�������ȷ���� �� ��


A��װ�â���������������ɫ����
B��װ�âڵĴ�������ƷӦ���Դ��������
C��װ�â������·������a������b��
D��װ�âܵ�������ӦΪ��2H+ + 2e�� = H2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ɽ��ʡ�����и�����ѧ��10���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��ͼ��ʾװ���У���֪������b���ص�������п�������ж���ȷ���� ( )
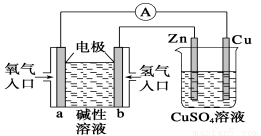
A����װ����Cu��Ϊ����
B��һ��ʱ���пƬ��������
C��b����Ӧ�ĵ缫��ӦʽΪH2��2e����2OH��===2H2O
D����ͭ���������仯Ϊ32 gʱ��a�������ĵ�O2�����Ϊ5.6 L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ɽ��ʡ�����и�����ѧ��10���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵����ȷ���� ( )
A���κλ�ѧ��Ӧ�������������ı仯
B�������¶Ȼ������������Ըı仯ѧ��Ӧ�ķ�Ӧ��
C����ѧ��Ӧ�е������仯��������������ʽ����
D��2CO(g)��O2(g)��2CO2(g) ��H<0����56g CO��32g O2�������������88g CO2�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ������ѧ�ڵ�һ��������ѧ�Ծ��������棩 ���ͣ�ѡ����
ʵ���ҴӺ��Ȼ��ơ�����ػ��Һ����ȡKNO3�Ĺ�������ͼ��ʾ�����з�����ȷ����
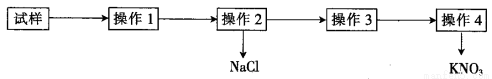
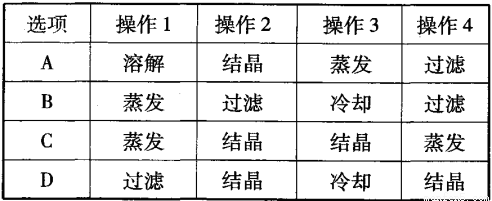
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����ϵڶ���ģ�⻯ѧ�Ծ��������棩 ���ͣ������
����ѧ����ѡ��2����ѧ�뼼����
ij���������̿�MnO2Լ70% ��A12O3������п��ZnSԼ80����FeS����ͬ����MnO2��Zn���ɵ��ԭ�ϣ���

��֪����A�� �Ļ��Һ��
�Ļ��Һ��
��IV�еĵ�ⷽ��ʽΪ��
��l��A�����ڻ�ԭ�������________________________��
��2������MnCO3��Zn2��OH��2CO3������ ________________________��
II��Ҫ���ȵ�ԭ���ǣ�____________________��C�Ļ�ѧʽ�� _______________��
��3���������г��õ�MnO2��Zn���⣬���ɵõ��ĸ���Ʒ��_________________��
��4����������������е���ģ�����ʯ�⣬�蹺��Ļ���ԭ����_____________��
��5��Ҫ��Na2SO4��Һ�еõ�â����Na2SO4��10H2O��������еIJ���������Ũ����___________�����ˡ�ϴ�ӡ�����ȡ�
��6��������MnO2��Zn�ĽǶȼ��㣬���̿����п��������ȴ�Լ��__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꼪��ʡ��һ���ڳ���ѧ���������棩 ���ͣ�ѡ����
������Һ�е�������Ũ����50 mL 1 mol��L��1��AlCl3��Һ��������Ũ����ȵ���
A��75 mL 2 mol��L��1��CaCl2 B��75 mL 1 mol��L��1��NH4Cl
C��150 mL 1 mol��L��1��NaCl D��150 mL 3 mol��L��1��KCl
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com