
����Ŀ�����������Ҫ�ɷ���Al2O3��������Fe2O3��SiO2�����ʣ�������������ȡ��������������ͼ��ʾ��
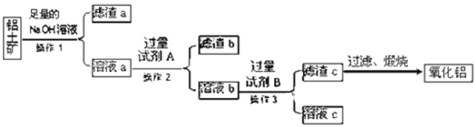
��1������1��������______������Ҫ����Ҫ���������У�______��
��2���Լ�A��______�����û�ѧʽ�ش𣩣�
��3����Һb���Լ�B��Ӧ�����ӷ���ʽΪ______________________��
��4����д���������������NaOH��Һ���������йط�Ӧ��ѧ����ʽ��______________��
��5��ijͬѧ��Ϊ��������Һa��ͨ����ǹ�����CO2��Ȼ��ֱ�ӽ��õ�������b���պ�Ҳ�ɵõ�Al2O3�����ҿ��Լ�������ȡ�����������̣�����Ϊ��ͬѧ�Ŀ���������______������������������������ǣ�______��������Ϊ������������Բ��ش�
��6��������ڵ����������Ʊ���������2Al2O3![]() 4Al+3O2�������ڱ�״��������2.24L����������÷�Ӧת�Ƶĵ�����Ϊ______��
4Al+3O2�������ڱ�״��������2.24L����������÷�Ӧת�Ƶĵ�����Ϊ______��
���𰸡����� ���������ձ���©�� HCl(��HNO3) H++NH3H2O=NH4++H2O ��Al3++3NH3��H2O =Al(OH)3��+3NH4+ Al2O3+2NaOH�T2NaAlO2+H2O��SiO2+2NaOH�TNa2SiO3+H2O ������ Al2O3�к���SiO2���� 0.4NA
��������
SiO2���������������������Ʒ�Ӧ���ܽ⣬����������м����������ƣ��õ���Һa�к���ƫ��������ӡ���������ӣ�����aΪ��������a��Һ�������A��Һ����ȥ��������ӣ�AΪ����Һ����ƫ���������ת��Ϊ�����ӣ�������Һb�У��ӹ���B������������������BΪ��ˮ�������������ȷֽ�����������ݴ˷������
(1)����1Ϊ���벻�������Һ�IJ�����Ϊ���ˣ�������������Ϊ������̨��©�������������ձ����ʴ�Ϊ�����ˣ���������©�����ձ���
����aΪ���������ʲ��ù��˵ķ������룬�ʴ�Ϊ�����ˣ���������©�����ձ���
(2)����������������ƣ��õ���Һa�к���ƫ��������ӡ���������ӣ��������A��Һ����ȥ��������ӣ���AΪ����������������Һ���ʴ�Ϊ��HCl��
(3)��Һb�к��������ӡ����������ᣬҪʹ�����ӳ���������������ˮ����ˮ�����ᷴӦ�����Ȼ�泥���ˮ���Ȼ�����Ӧ�����Ȼ�狀�����������������Ӧ�ķ���ʽΪHCl+NH3H2O=NH4Cl+H2O��AlCl3+3NH3H2O=Al(OH)3��+3NH4Cl�����ӷ���ʽΪ��H++NH3H2O=NH4++H2O��Al3++3NH3��H2O =Al(OH)3��+3NH4+���ʴ�Ϊ��H++NH3H2O=NH4++H2O��Al3++3NH3��H2O =Al(OH)3��+3NH4+��
(4)���������Ҫ�ɷ���Al2O3��������Fe2O3��SiO2�����ʣ�����������������������������Ʒ�Ӧ����ƫ�����ƺ����ƣ���Ӧ�ķ���ʽΪ��Al2O3+2NaOH�T2NaAlO2+H2O��SiO2+2NaOH�TNa2SiO3+H2O���ʴ�Ϊ��Al2O3+2NaOH�T2NaAlO2+H2O��SiO2+2NaOH�TNa2SiO3+H2O��
(5)��Һa�к���ƫ��������ӡ���������ӣ�������Һa��ͨ�������CO2���������������������������Ȼ��ֱ�ӽ��õ�������b���պ�õ��Ĺ����к����������Ͷ������裬��Al2O3�к���SiO2���ʣ��ʴ�Ϊ����������Al2O3�к���SiO2���ʣ�
(6)��״���£�2.24L���������ʵ���Ϊ![]() =0.1mol��������ڵ����������Ʊ�����������������������������������������ӦΪ2O2--4e-=O2����ת��0.4mol���ӣ���ת�Ƶ���0.4NA���ʴ�Ϊ��0.4NA��
=0.1mol��������ڵ����������Ʊ�����������������������������������������ӦΪ2O2--4e-=O2����ת��0.4mol���ӣ���ת�Ƶ���0.4NA���ʴ�Ϊ��0.4NA��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��FeSe��MgB2��Nb3Al�ȳ������Ͼ��й㷺Ӧ��ǰ����
��1��Fe2+��̬�۵��ӵĹ����ʾʽ���۵����Ų�ͼ��__��Se��Mg��B����Ԫ�صĵ縺�Դ�С˳����__��
��2������״̬���Զ��۷��Ӵ��ڵ�A1Cl3�Ľṹʽ��___������A1ԭ�ӵ��ӻ���ʽ��___�������а˸�ԭ��___(ѡ������������������)��ͬһƽ���ϣ��÷�����____(ѡ���������������Ǽ�����)���ӡ�
��3���Ʊ�FeSe����������Li0.6(NH2)0.2(NH3)0.8Fe2Se2�������轫���������Һ�����Ӷ��Ƶþ��кܸ߷�Ӧ���ԵĽ���������Һ����ӦΪ��Li+(m+n)NH3��X+e-(NH3)n��
��X�Ļ�ѧʽΪ____��
��NH3�ļ۲���ӶԻ���ģ���� ____��
��4��MgB2����ṹ�е�Bԭ�Ӳ��������ʯī�IJ�״�ṹ���ұ��������ŵ�Mgԭ�Ӳ����,Bԭ��λ��Mgԭ����ɵ������������ġ���֪��ƽ����Mgԭ�Ӽ������˼��Ϊacm��ƽ���Mgԭ�Ӽ������˼��Ϊbcm�������ӵ�����ΪNA��
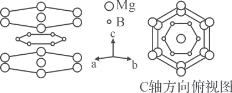
��Bԭ�Ӳ���Ԫ���д��ڶ�λ��Bԭ�Ӻ˼��Ϊ____cm��
��MgB2������ܶ���____g��cm-3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�л���Ľṹ��ͼ��ʾ������˵����ȷ����
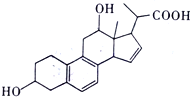
A. ���л���ķ���ʽΪC21H24O4
B. ���л��ﹲ�����ֹ����ţ��ֱ��ǣ��ǻ����Ȼ���������̼̼˫��
C. ���л����������NaOH��NaHCO3�����ʵ�����Ϊ1:1
D. 1mol ���л��������������Ʒ�Ӧ������33.6L����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ۺ�����CO2��CO�Թ�����̼�������Ҫ���塣CO��H2�ڴ��������·������·�Ӧ��CO(g)��2H2(g) ![]() CH3OH(g)���Դ˷�Ӧ���������о���ij�¶�����һ��ѹ�����зֱ����2.4 mol CO��2 mol H2���ﵽƽ��ʱ�������Ϊ4 L���Һ���0.8 mol CH3OH(g)��д����Ҫ�ļ�����̽��м��㣺
CH3OH(g)���Դ˷�Ӧ���������о���ij�¶�����һ��ѹ�����зֱ����2.4 mol CO��2 mol H2���ﵽƽ��ʱ�������Ϊ4 L���Һ���0.8 mol CH3OH(g)��д����Ҫ�ļ�����̽��м��㣺
(1)�÷�Ӧ�Ļ�ѧƽ�ⳣ��_____________________��
(2)��ʱ����������ͨ��0.7 mol CO���壬�ж�ƽ���ƶ��ķ���________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ����ú����IJ���WΪ����ԭ�Ϻϳ�һϵ�л�����Ʒ������������(���ֲ��������ʡ��):

��֪������Ϣ����:
��1mol������W��50mol������
��![]() ��
��
��![]() (�������ױ�����)
(�������ױ�����)
��K�ķ���ʽΪC7H6O2����˴Ź�����������4���塣
��ش���������:
��1��X������Ϊ_________��Y�����������ŵ�����Ϊ_________ ��
��2����Ӧ��������Ϊ_________�����ķ�Ӧ����________��
��3����Ӧ���Ļ�ѧ����ʽΪ_________��
��4��Z�Ľṹ��ʽΪ_________ ��
��5��K��ͬ���칹��M���ܷ���ˮ�ⷴӦ�����ܷ���������Ӧ��M������������Һ�з���ˮ�ⷴӦ�Ļ�ѧ����ʽΪ__________��
��6�� �ж���ͬ���칹�壬д����������3��������ͬ���칹��Ľṹ��ʽ_________��
�ж���ͬ���칹�壬д����������3��������ͬ���칹��Ľṹ��ʽ_________��
���ܷ���������Ӧ������ˮ�⡣
��ÿĦ��ͬ���칹���������2molNaOH��
��������һ�ȴ���ֻ�����֡�
��7����������ͼ����ʽд����T�Ʊ� �Ĺ���(���Լ���ѡ):������
�Ĺ���(���Լ���ѡ):������ ��_____________________
��_____________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��д����˿������ͭ��Һ�Ļ�ѧ����ʽ������˫���ű�����ӵ�ת�ơ���ѧ����ʽ�� _______________����������__________ ����ԭ���� __________���������___________ ����ԭ���________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������(NaN3)�ǰ�ɫ����ϵ���壬�綾��������ˮ����HN3(������ᣬ������������Ƶ�����)�����Ρ��ش��������⣺
(1)ʵ���ҿ���NaN3�ֽ�(�������ֵ���)�Ʊ��ߴ�N2����֪NAΪ�����ӵ�������ֵ��ÿ����1 mol N2ת�Ƶ�����Ϊ___________��
(2)NaN3��ϡ���ᷴӦ��HN3�����ӷ���ʽΪ______________��
(3)NaNH2��N2O��210��220��ʱ��Ӧ���Ʊ�NaN3��ͬʱ�ų���ʹʪ���ɫʯ����ֽ���������壬�÷�Ӧ�Ļ�ѧ����ʽΪ_____________________��
(4)�ⶨ��ҵƷ����������NaN3����������ʵ�鲽�����£�
(I)ȷ��������m g����������NaOH���ձ��У�������������ˮ�ܽ⣬ת����250mL����ƿ�ж��ݣ�
(II)ȷ��ȡ��õ���Һ20.00mL����ƿ�У���������V1 mL c1 mol��L��1(NH4)2Ce(NO3)6��Һ����ַ�Ӧ������ˮϡ�ͣ�����5 mLŨ���ᣬ��2��������ָʾ������c2 mol��L��1(NH4)2Fe(SO4)2����Һ�ζ�����Һ�ɵ���ɫ��Ϊ�ƺ�ɫ(Ce4����Fe2����Ce3����Fe3��)�����ı���ҺV2 mL��
��NaN3��(NH4)2Ce(NO3)6��Һ��Ӧ����������ΪN2����ԭ����ΪCe(NO3)3�������ӷ���ʽΪ______��
������Ʒ��NaN3����������Ϊ__________(�г��������ʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪AΪ�����Ľ������ʣ�����������ʾ��ת����ϵ�ش��������⣺
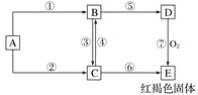
(1)д���������ʵĻ�ѧʽ��
A________��B_______��C_______��D_________��E__________��
(2)��Ӧ�۵����ӷ���ʽΪ_______________��
��Ӧ�ܵ����ӷ���ʽΪ___________��
��Ӧ�ߵĻ�ѧ����ʽΪ_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ס��ҡ����������������У��ס��ҡ�����������ͬ��ij��Ԫ�أ�����֮���������ת����ϵ����![]() ��
��![]() ���������й����ʵ��ƶϴ������[��֪Al3++3AlO2-+6H2O=4Al(OH)3��]
���������й����ʵ��ƶϴ������[��֪Al3++3AlO2-+6H2O=4Al(OH)3��]
A.����Ϊ��̿��������O2
B.����ΪAlCl3��Һ��������KOH��Һ
C.����ΪCu��������Cl2
D.����ΪNaOH��Һ��������CO2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com