
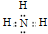 ��N��H��ɵ����ӻ�����һ������笠����ӣ�
��N��H��ɵ����ӻ�����һ������笠����ӣ�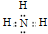 ��NH4H��
��NH4H��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��
| ||
B��
| ||
C��
| ||
D��
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��0.3mol |
| B��0.5mol |
| C��0.15mol |
| D��0.2mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��NaOH��H2SO4����NH4��2SO4 |
| B��MgO��Na2SO4��HNO3 |
| C��Na2O2��KOH��Na3PO4 |
| D��HCl��Na2O��MgCl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��n��a% | ||
| B��n-m | ||
C��
| ||
| D����n-m����a% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��25��ʱ��pH=1��1.0 L H2SO4��Һ�к��е�H+����ĿΪ0.2NA |
| B����״���£�2.24 L Cl2�����ϡNaOH��Һ��Ӧ��ת�Ƶĵ�������Ϊ0.2NA |
| C�������£�21.0 g��ϩ�Ͷ�ϩ�Ļ�������к��е�̼ԭ����ĿΪ1.5NA |
| D��ȡ0.5 L 0.2 mol?L-1�Ȼ�����Һ��������Һ����0.1 NA��Fe3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����������Һ�м������������������Һ��Al3++2SO42-+2Ba2++4OH-�T2BaSO4��+AlO2-+2H2O |
| B��ϡHNO3��Һ��������ˮ��FeS���壺FeS+2H+�TFe2++H2S�� |
| C��̼����þ����������ʯ��ˮ��Ӧ��Ca2++2OH-+2HCO3-�TCaCO3��+CO32-+2H2O |
| D��ǿ����Һ�У�����������Fe��OH��3��3ClO-+2Fe��OH��2�T2FeO42-+3Cl-+H2O+4H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com