
| [H+]?[HCO3-] |
| [H2CO3] |
| [H+]?[HCO3-] |
| [H2CO3] |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������Һ�в������Ӽ����㣺c��CH3COO-��=c��Na+�� �����Һһ�������� |
| B����Һ����������֮�䲻�������㣺c��Na+����c��OH-����c��CH3COO-����c��H+�� |
| C������Һ�����ʽ�ΪCH3COONa�������Ӽ�һ�����㣺c��Na+����c��CH3COO-����c��OH-����c��H+�� |
| D������Һ�����Ӽ����㣺c��CH3COO-����c�� Na+����c��H+����c��OH-��������Һ������һ����CH3COONa��CH3COOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����������CuS���� |
| B����������FeS���� |
| C����������FeCl2���� |
| D����������CuCl2���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������Һ�п��ܺ�K+ |
| B������Һ�п϶�����NO3?��SO42-��NH4+��CO32- |
| C������Һ��һ������NH4+ |
| D������Һ��һ����K+����c��K+����0.1mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ����ʵ | �������� | |
| �� | 18gij�л�����ȫȼ�գ�ֻ�õ�26.4gCO2��10.8gH2O | ���ʽ |
| �� | ���л������Է�������Ϊ180 | ����ʽ |
| �� | һ�������£�180g���л�����������ȫ�ۻ�������������������Ϊ390g | һ���������� |
| �� | ���л�����Է���������Ӧ | �������� |
| �� | ���л�����Ա���ԭΪֱ�������� | ���ӽṹ�� |
| ���л���ṹ��ʽ | ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ѹǿ/MPa �������/% �¶�/�� | 1.0 | 2.0 | 3.0 |
| 810 | 54.0 | a | b |
| 915 | c | 75.0 | d |
| 1000 | e | f | 83.0 |
| A��b��f |
| B��915�桢2.0MPaʱE��ת����Ϊ50% |
| C���÷�Ӧ�ġ�S��0 |
| D��K��1000�棩��K��810�棩 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

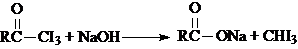
| A��CH3CHO |
| B��CH3CH2CHO |
| C��CH3CH2COCH2CH3 |
| D��CH3COCH2CH3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com