
”¾ĢāÄæ”æŌ×ÓŠņŹżŅĄ“ĪŌö“óµÄX”¢Y”¢Z”¢Q”¢EĪåÖÖŌŖĖŲÖŠ£¬XŌŖĖŲŌ×ÓŗĖĶāÓŠČżÖÖ²»Ķ¬µÄÄܼ¶ĒŅø÷øöÄܼ¶ĖłĢī³äµÄµē×ÓŹżĻąĶ¬£¬ZŹĒµŲæĒÄŚŗ¬Įæ£ØÖŹĮæ·ÖŹż£©×īøßµÄŌŖĖŲ£¬QŌ×ÓŗĖĶāµÄM²ćÖŠÖ»ÓŠĮ½¶Ō³É¶Ōµē×Ó£¬EŌŖĖŲŌ×ÓŠņŹżĪŖ29”£
ÓĆŌŖĖŲ·ūŗÅ»ņ»ÆѧŹ½»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)YŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆĪŖ__________________”£
(2)ŅŃÖŖYZ2+ÓėXO2»„ĪŖµČµē×ÓĢ壬Ōņ1mol YZ2+ÖŠŗ¬ÓŠ¦Š¼üŹżÄæĪŖ___________”£
(3)X”¢ZÓėĒāŌŖĖŲæÉŠĪ³É»ÆŗĻĪļXH2Z£¬XH2Z·Ö×ÓÖŠXµÄŌӻƷ½Ź½ĪŖ_________________”£
(4)EŌ×ÓµÄŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ__________£»EÓŠæɱä¼ŪĢ¬£¬ĖüµÄij¼ŪĢ¬µÄĄė×ÓÓėZµÄŅõĄė×ÓŠĪ³É¾§ĢåµÄ¾§°ūČēĶ¼ĖłŹ¾£¬øĆ¼ŪĢ¬µÄ»ÆѧŹ½ĪŖ____________”£
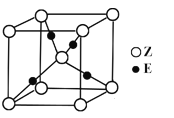
(5)ŃõŌŖĖŲŗĶÄĘŌŖĖŲÄܹ»ŠĪ³É»ÆŗĻĪļF£¬Ę侧°ū½į¹¹ČēĶ¼ĖłŹ¾£ØĮ¢·½Ģ徧°ū£©£¬¾§ĢåµÄĆܶČĪŖ¦Ńg”¤”¤cm-3£¬ĮŠŹ½¼ĘĖć¾§°ūµÄ±ß³¤ĪŖa=______________cm£ØŅŖĒóĮŠ“śŹżŹ½£©”£

”¾“š°ø”æ µŚ¶žÖÜĘŚ µŚVA×å 2NA»ņ1.204”Į1024 sp2ŌÓ»Æ 1s22s22p63s13p23d63d104s1»ņ[Ar]3d104s1 Cu2O 
”¾½āĪö”æXŌŖĖŲŌ×ÓŗĖĶāÓŠČżÖÖ²»Ķ¬µÄÄܼ¶ĒŅø÷øöÄܼ¶ĖłĢī³äµÄµē×ÓŹżĻąĶ¬£¬ŌņXĪŖC£¬ZŹĒµŲæĒÖŠŗ¬ÓŠ×īøßµÄŌŖĖŲ£¬¼“ZĪŖO£¬£¬ŅņĪŖŌ×ÓŠņŹżŅĄ“ĪŌö“ó£¬ŌņYĪŖN£¬QŌ×ÓŗĖĶāµÄM²ćÖŠÖ»ÓŠĮ½¶Ō³É¶Ōµē×Ó£¬¼“QĪŖS£¬EµÄŌ×ÓŠņŹżĪŖ29£¬ŌņEĪŖCu£¬£Ø1£©æ¼²éŌŖĖŲŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĪ»ÖĆ£¬YŹĒN£¬Ī»ÓŚµŚ¶žÖÜĘŚVA×壻£Ø2£©æ¼²éµČµē×ÓĢåŗĶ¦Š¼üÅŠ¶Ļ£¬YZ2£«ĪŖNO2£«£¬ÓėCO2»„ĪŖµČµē×ÓĢ壬µČµē×ÓĢåµÄ½į¹¹ĻąĖĘ£¬CO2µÄ½į¹¹Ź½ĪŖO=C=O£¬Ņņ“Ė1molNO2£«ÖŠŗ¬ÓŠ¦Š¼üµÄŹżÄæĪŖ2NAøö£»£Ø3£©æ¼²éŌÓ»ÆĄąŠĶ£¬ŠĪ³ÉµÄ»ÆŗĻĪļŹĒHCHO£¬ĘäÖŠĢ¼Ō×ÓŹĒsp2Ōӻƣ»£Ø4£©æ¼²éŗĖĶāµē×ÓÅŲ¼Ź½£¬Ķعż¾§°ūµÄ½į¹¹Č·¶Ø»ÆѧŹ½£¬CuĪ»ÓŚµŚĖÄÖÜĘŚIB×壬ŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ1s22s22p63s13p23d63d104s1»ņ[Ar]3d104s1 £»OŌ×ÓĪ»ÓŚ¶„µćŗĶĢåŠÄ£¬øöŹżĪŖ8”Į1/8£«1=2£¬CuČ«²æĪ»ÓŚĢåŠÄ£¬Ņņ“Ė»ÆѧŹ½ĪŖCu2O£»£Ø5£©æ¼²é¾§°ūµÄ¼ĘĖć£¬ŗĖĶāµē×ÓÅŲ¼ĻąĶ¬Ź±£¬°ė¾¶Ėę×ÅŌ×ÓŠņŹżŌö“ó¶ų¼õŠ”£¬¼“ŃõŌŖĖŲĪ»ÓŚ¶„µćŗĶĆęŠÄ£¬øöŹżĪŖ8”Į1/8£«6”Į1/2=4£¬NaŌŖĖŲĪ»ÓŚ¾§°ūÄŚ£¬ÓŠ8øö£¬Ņņ“Ė»ÆѧŹ½ĪŖNa2O£¬¾§°ūµÄÖŹĮæĪŖ4”Į62/NAg£¬¾§°ūµÄĢå»żĪŖa3cm3£¬øł¾ŻĆܶȵĶØŅ壬ӊ¦Ń=4”Į62/£ØNA”Įa3£©£¬¼“±ß³¤ĪŖ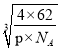 cm”£
cmӣ



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æµā¼°Ęä»ÆŗĻĪļŌŚæĘŃŠ”¢Éś»īµČ·½ĆęÓŠ¹ć·ŗÓĆĶ¾”£»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ŗ£“ųÖŠø»ŗ¬µā£¬°“ČēĻĀŹµŃéĮ÷³ĢæɶŌŗ£“ųÖŠµāµÄŗ¬Įæ½ųŠŠ²ā¶Ø”£

Č”0.0100 mol/LµÄAgNO3±ź×¼ČÜŅŗ×°Čė×ŲÉ«µĪ¶Ø¹Ü£¬Č”100.00 mLŗ£“ų½žČ”ŌŅŗÖĮµĪ¶Ø³Ų£¬ÓƵēŹĘµĪ¶Ø·Ø²ā¶Øµāŗ¬Į攣²āµĆµÄµē¶ÆŹĘ(E) ·“Ó³ČÜŅŗÖŠc(I”„)µÄ±ä»Æ£¬²æ·ÖŹż¾ŻČēĻĀ±ķ£ŗ
V(AgNO3)/mL | 15.00 | 19.00 | 19.80 | 19.98 | 20.00 | 20.02 | 21.00 | 23.00 | 25.00 |
E/mV | £225 | £200 | £150 | £100 | 50.0 | 175 | 275 | 300 | 325 |
¢Ł×ĘÉÕŗ£“ųŹ±£¬³żŠčŅŖŪįŪöĶā£¬»¹ŠčŅŖÓƵ½µÄŹµŃéŅĒĘ÷ŹĒ____________ (ĢīŠņŗÅ)”£
a£®ÉÕ± b£®Čż½Å¼Ü c£®ĪĀ¶Č¼Ę d£®ÄąČż½Ē e£®¾Ę¾«µĘ f£®ŪįŪöĒÆ
¢ŚŹ¹ÓĆ×ŲÉ«µĪ¶Ø¹ÜµÄŌŅņŹĒ_____________________”£
¢Ūøł¾Ż±ķÖŠŹż¾Ż£¬¼ĘĖćŗ£“ųÖŠµāµÄ°Ł·Öŗ¬ĮæĪŖ____________”£
(2)”°“óĻóµÄŃĄøą”±ŹĒÖųĆū»ÆѧŹµŃéÖ®Ņ»£¬Ę䏵Ńé·½·ØŹĒ½«ÅØĖõµÄ¹żŃõ»ÆĒāČÜŅŗÓė·ŹŌķŅŗ»ģŗĻ£¬ŌŁµĪ¼ÓÉŁĮæµā»Æ¼ŲČÜŅŗ£¬¼“æɹŪ²ģµ½ÅŻÄדĪļÖŹĻńÅēČŖŅ»ŃłÅēÓæ¶ų³ö”£
ŅŃÖŖ£ŗ2H2O2(l)=2H2O(l)+O2(g) ”÷H=£196kJ/mol£¬»ī»ÆÄÜEa=76kJ/mol£¬ČōÓĆI£“ß»ÆŹ±»ī»ÆÄÜEa”Æ=57kJ/mol”£
¢ŁŌŚH2O2ČÜŅŗÖŠ¼ÓČėKIČÜŅŗ×÷“߻ƼĮ£¬·“Ó¦¹ż³ĢÖŠ·¢ÉśI£ÓėIO£Ö®¼äµÄ×Ŗ»Æ£¬ĒėŅĄ“ĪŠ“³ö·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
·“Ó¦¼×£ŗ______________________£»
·“Ó¦ŅŅ£ŗ______________________”£
¢Ś·“Ó¦¼×ĪŖĪüČČ·“Ó¦£¬ĒŅ¼×µÄ·“Ó¦ĖŁĀŹŠ”ÓŚŅŅµÄ·“Ó¦ĖŁĀŹ£¬ŌŚĻĀĶ¼ÖŠ»³öŌŚH2O2ČÜŅŗÖŠ¼ÓČėKIŗ󣬷“Ó¦¹ż³ĢµÄÄÜĮæ±ä»ÆĶ¼”£_______

(3)HI²»ĪČ¶Ø£¬ĘäĖ®ČÜŅŗ¾ßÓŠĒæĖįŠŌ”£ĻÖÓĆ0.lmol/LKIČÜŅŗ”¢NH4I¹ĢĢ唢pHŹŌÖ½Éč¼ĘŹµŃéŃéÖ¤ÉĻŹöŠŌÖŹ”£¼ņŹöŹµŃé·½°ø”£
¢ŁĒæĖįŠŌ£ŗ__________________________”£
¢Ś²»ĪČ¶ØŠŌ£ŗ___________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŌŚ10 LĆܱÕČŻĘ÷ÖŠ£¬1 mol AŗĶ3 mol BŌŚŅ»¶ØĢõ¼žĻĀ·“Ó¦£ŗA(g)£«xB(g)![]() 2C(g)£¬2 minŗó·“Ó¦“ļµ½Ę½ŗāŹ±£¬²āµĆ»ģŗĻĘųĢå¹²3.4 mol£¬Éś³É0.4 mol C£¬ŌņĻĀĮŠ¼ĘĖć½į¹ūÕżČ·µÄŹĒ
2C(g)£¬2 minŗó·“Ó¦“ļµ½Ę½ŗāŹ±£¬²āµĆ»ģŗĻĘųĢå¹²3.4 mol£¬Éś³É0.4 mol C£¬ŌņĻĀĮŠ¼ĘĖć½į¹ūÕżČ·µÄŹĒ
A. Ę½ŗāŹ±£¬ĪļÖŹµÄĮæÖ®±Čn(A)”Ćn(B)”Ćn(C)£½2”Ć11”Ć4
B. xÖµµČÓŚ3
C. AµÄ×Ŗ»ÆĀŹĪŖ20%
D. BµÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ0.4 mol”¤L£1”¤min£1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æµķ·Ū KI ŹŌÖ½²»ÄÜÓĆĄ“¼ģŃé£ŗ
A. Cl2 B. Br2 C. I2 D. I-
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŹµŃéŹŅĄļĄūÓĆŅŌĻĀ·“Ó¦ÖĘȔɣĮæµŖĘų£ŗNaNO2£«NH4Cl===NaCl£«N2”ü£«2H2O”£¹ŲÓŚøĆ·“Ó¦µÄĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø £©
A£®ĆæÉś³É1 mol N2×ŖŅʵĵē×ÓµÄĪļÖŹµÄĮæĪŖ6 mol
B£®N2¼ČŹĒŃõ»Æ¼Į£¬ÓÖŹĒ»¹Ō¼Į
C£®NH4ClÖŠµÄµŖŌŖĖŲ±»»¹Ō
D£®NaNO2ŹĒŃõ»Æ¼Į
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æµē»ÆѧĘųĆō“«øŠĘ÷æÉÓĆÓŚ¼ą²ā»·¾³ÖŠNH3µÄŗ¬Į棬Ę乤×÷ŌĄķŹ¾ŅāĶ¼ČēĻĀ”£ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ( )
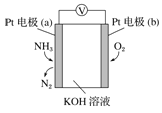
A£®ČÜŅŗÖŠOH-Ļņµē¼«aŅʶÆ
B£®O2ŌŚµē¼«bÉĻ·¢Éś»¹Ō·“Ó¦
C£®·“Ó¦ĻūŗĵÄNH3ÓėO2µÄĪļÖŹµÄĮæÖ®±ČĪŖ 4:5
D£®µē¼« a µÄ·“Ó¦Ź½ĪŖ 2NH3-6e-+6OH-£½N2+6H2O
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ“ÓĻĀĮŠ»łĶÅ£ŗ-CH3”¢-OH”¢-COOH”¢-C6H6£¬Ļą»„Į½Į½×é³ÉµÄÓŠ»śĪļÓŠ
A. 3øö B. 4øö C. 5øö D. 6øö
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠĪļÖŹÖŠŹōÓŚµē½āÖŹµÄŹĒ
A£®CO2 B£®BaSO4 C£®Zn D£®Ź³ŃĪĖ®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻõĖįŹĒŅ»ÖÖÖŲŅŖµÄ»Æ¹¤ŌĮĻ£¬¹¤ŅµÉĻÉś²śĻõĖįµÄÖ÷ŅŖ¹ż³ĢÖŠ£ŗ£Ø1£©·“Ó¦N2£Øg£©+3H2£Øg£©![]() 2NH3£Øg£©”÷H<0 £»ŗćĪĀŹ±£¬½«N2ŗĶH2µÄ»ģŗĻĘųĢå³äČė2LĆܱÕČŻĘ÷ÖŠ£¬10·ÖÖÓŗó·“Ó¦“ļµ½Ę½ŗāĻĀĮŠĶ¼ĻńÄÜÕżČ·±ķŹ¾øĆ¹ż³ĢÖŠĻą¹ŲĮæµÄ±ä»ÆµÄŹĒ__________”££ØŃ”Ģī×ÖÄø£©”£
2NH3£Øg£©”÷H<0 £»ŗćĪĀŹ±£¬½«N2ŗĶH2µÄ»ģŗĻĘųĢå³äČė2LĆܱÕČŻĘ÷ÖŠ£¬10·ÖÖÓŗó·“Ó¦“ļµ½Ę½ŗāĻĀĮŠĶ¼ĻńÄÜÕżČ·±ķŹ¾øĆ¹ż³ĢÖŠĻą¹ŲĮæµÄ±ä»ÆµÄŹĒ__________”££ØŃ”Ģī×ÖÄø£©”£

(2)Ņ»¶ØĪĀ¶ČĻĀ£¬ŌŚĆܱÕČŻĘ÷ÖŠ³äČė1molN2ŗĶ3molH2·¢Éś·“Ó¦”£ČōČŻĘ÷ČŻ»żŗć¶Ø£¬“ļµ½Ę½ŗāדĢ¬Ź±£¬ĘųĢåµÄ×ÜĪļÖŹµÄĮæŹĒŌĄ“µÄ7/10£¬ŌņN2µÄ×Ŗ»ÆĀŹa1=________£»
ČōĻņøĆČŻĘ÷ÖŠŌŁ¼ÓČė1molN2ŗĶ3molH2£¬“ļµ½Ę½ŗāדĢ¬Ź±£¬N2µÄ×Ŗ»ÆĀŹĪŖa2£¬Ōņa2________a1£ØĢī”°>”±”¢”°<”±»ņ”°=”±£©”£
£Ø3£©2NO£Øg£©+O2£Øg£©![]() 2NO2(g)”£ŌŚĘäĖūĢõ¼žĻąĶ¬Ź±£¬·Ö±š²āµĆNOµÄĘ½ŗā×Ŗ»ÆĀŹŌŚ²»Ķ¬Ń¹Ēæ£ØP1”¢P2£©ĻĀĪĀ¶Č±ä»ÆµÄĒśĻߣØČēĶ¼£©”£
2NO2(g)”£ŌŚĘäĖūĢõ¼žĻąĶ¬Ź±£¬·Ö±š²āµĆNOµÄĘ½ŗā×Ŗ»ÆĀŹŌŚ²»Ķ¬Ń¹Ēæ£ØP1”¢P2£©ĻĀĪĀ¶Č±ä»ÆµÄĒśĻߣØČēĶ¼£©”£

¢Ł±Č½ĻP1”¢P2µÄ“󊔹ŲĻµ£ŗP1____P2£ØĢī”°>”±”¢”°<”±»ņ”°=”±£©”£
¢ŚĖęĪĀ¶ČÉżøߣ¬øĆ·“Ó¦Ę½ŗā³£Źż±ä»ÆµÄĒ÷ŹĘŹĒ__________”£
£Ø4£©ĻõĖį³§³£ÓĆČēĻĀ2ÖÖ·½·Ø“¦ĄķĪ²Ęų”£
¢Ł“߻ƻ¹Ō·Ø£ŗ“߻ƼĮ“ęŌŚŹ±ÓĆH2½«NO2»¹ŌĪŖN2”£
ŅŃÖŖ£ŗ2H2£Øg£©+O2£Øg£©=2H2O£Øg£©”÷H=-483.6kJ/mol
N2£Øg£©+2O2£Øg£©=2NO2£Øg£©”÷H=+67.7kJ/mol
ŌņH2»¹ŌNO2Éś³ÉĖ®ÕōĘų·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ŹĒ________________________________”£
¢Ś¼īŅŗĪüŹÕ·Ø£ŗÓĆNa2CO3ČÜŅŗĪüŹÕNO2Éś³ÉCO2”£ČōĆæ4.6gNO2ŗĶNa2CO3ČÜŅŗ·“Ó¦Ź±×ŖŅʵē×ÓŹżĪŖ0.05mol£¬Ōņ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ___________________________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com