
��6�֣���ҵ����NH3��������ˮ��ԭ�ϣ��ϳɻ��������100 t��
��1����ҪNH3 ��,��Ҫ���� �֡�
��2����NH3��NOת����Ϊ96%��NO��HNO3ת����Ϊ92%����ҪNH3 �֣�������λС������
����������1����ҵ��������淋ķ�ӦʽΪNH3��HNO3=NH4NO3������淋����ʵ�����![]() �����Ը���ԭ���غ��֪��Ҫ���������ʵ�����
�����Ը���ԭ���غ��֪��Ҫ���������ʵ�����![]() ����������
����������![]() ����Ϊ42.5�֡�����һ��İ��������������ᣬ��һ��������������李���������ķ�ӦΪ4NH3��5O2
����Ϊ42.5�֡�����һ��İ��������������ᣬ��һ��������������李���������ķ�ӦΪ4NH3��5O24NO��6H2O��4NO��3O2��2H2O=4HNO3����NH3��O2=HNO3��H2O��������Ҫ���������ʵ�����
����������
��32g/mol=80��106g����������80�֡�
��2�����ݣ�1���ķ�����֪�����������������Ҫ������21.25�֣����Ը����й�ת���ʿɼ��㣬��������ʵ����Ҫ������![]() ������������Ҫ�İ�����24.06t��21.25t��45.31t��
������������Ҫ�İ�����24.06t��21.25t��45.31t��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��������ʡ����������ѧ2011��2012ѧ���һ��ѧ�ڵ�һ�ο��Ի�ѧ���� ���ͣ�038
��ҵ����NH3��������ˮ��ԭ�ϣ��ϳɻ��������100 t��
(1)��ҪNH3________�֣���Ҫ����________�֣�
(2)��NH3��NOת����Ϊ96����NO��HNO3ת����Ϊ92������ҪNH3________��(������λС��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���dz����⡤�̲�ȫ��ȫ�������л�ѧ������1��(ɽ����) ɽ���� ���ͣ�038
��ҵ����NH3��������ˮ��ԭ�ϣ��ϳɻ�������泥�
(1)��Ҫ��100 t����泥���NH3���ٶ֣������е��������ٶ֣�ˮ���ٶ֣�
(2)��NH3��NO��ת����Ϊ96����NOת��ΪHNO3��ת����Ϊ92��������100 t�������NH3���ٶ֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012�����������������ѧ��һ��ѧ�ڵ�һ�νμ�⻯ѧ�Ծ����������� ���ͣ�������
��6�֣���ҵ����NH3��������ˮ��ԭ�ϣ��ϳɻ��������100 t��
��1����ҪNH3 ��,��Ҫ���� �֡�
��2����NH3��NOת����Ϊ96%��NO��HNO3ת����Ϊ92%����ҪNH3 �֣�������λС������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012�����������������ѧ��һ��ѧ�ڵ�һ�νμ�⻯ѧ�Ծ��������棩 ���ͣ�������
��6�֣���ҵ����NH3��������ˮ��ԭ�ϣ��ϳɻ��������100 t��
��1����ҪNH3 ��,��Ҫ���� �֡�
��2����NH3��NOת����Ϊ96%��NO��HNO3ת����Ϊ92%����ҪNH3 �֣�������λС������
����������1����ҵ��������淋ķ�ӦʽΪNH3��HNO3=NH4NO3������淋����ʵ�����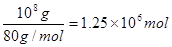 �����Ը���ԭ���غ��֪��Ҫ���������ʵ�����
�����Ը���ԭ���غ��֪��Ҫ���������ʵ����� ����������
���������� ����Ϊ42.5�֡�����һ��İ��������������ᣬ��һ��������������李���������ķ�ӦΪ4NH3��5O2
����Ϊ42.5�֡�����һ��İ��������������ᣬ��һ��������������李���������ķ�ӦΪ4NH3��5O2 4NO��6H2O��4NO��3O2��2H2O=4HNO3����NH3��O2=HNO3��H2O��������Ҫ���������ʵ�����
4NO��6H2O��4NO��3O2��2H2O=4HNO3����NH3��O2=HNO3��H2O��������Ҫ���������ʵ����� ����������
���������� ��32g/mol=80��106g����������80�֡�
��32g/mol=80��106g����������80�֡�
��2�����ݣ�1���ķ�����֪�����������������Ҫ������21.25�֣����Ը����й�ת���ʿɼ��㣬��������ʵ����Ҫ������ ������������Ҫ�İ�����24.06t��21.25t��45.31t��
������������Ҫ�İ�����24.06t��21.25t��45.31t��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com