
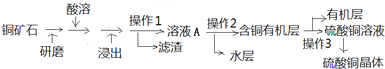
| 100mL |
| 20mL |
| 1.25bc |
| a |
| 5bc |
| 4a |
| 5bc |
| 4a |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�� | �����Ϣ |
| X | X����������Ϊ24��������Ϊ12�ĺ��� |
| Y | Y�ĵ����dz��õİ뵼����� |
| Z | Z�ĵ�����һ�ֲ�����ˮ�ĵ���ɫ���壬������CS2 |
| W | �۵����Ų�ʽ3d104s1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����Һ��SO42-��Ũ����0.3 mol/L |
| B����Һ��һ������A13+��NH4+ |
| C��һ��������Mg2+�����ܴ���A13+ |
| D��һ������Cl- ���ܺ�CO32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
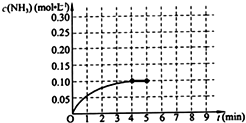 ��һ�̶��ݻ�Ϊ2L���ܱ������ڼ���0.2mol��N2��0.6mol��H2����һ�������·������·�Ӧ��N2��g��+3H2��g��?2NH3��g����H��0����Ӧ��NH3�����ʵ���Ũ�ȵı仯�����ͼ��ʾ����ش��������⣺
��һ�̶��ݻ�Ϊ2L���ܱ������ڼ���0.2mol��N2��0.6mol��H2����һ�������·������·�Ӧ��N2��g��+3H2��g��?2NH3��g����H��0����Ӧ��NH3�����ʵ���Ũ�ȵı仯�����ͼ��ʾ����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com