
��12�֣���ˮ�д�������ƽ�⣺Cl2 +H2O ? HCl+HClO������ˮ�м����������ʣ��ش�����⣺
��1����������CaCO3(s)��������Ӧ�����ӷ���ʽ��ʾΪ__________________��ƽ����________�ƶ��������������������ͬ����HClO��Ũ��______�����������С�����䡱����ͬ����
��2����������NaCl(s)��ƽ����_________�ƶ���HClO��Ũ��___________��
��3����������NaOH(s)��ƽ����__________�ƶ���HClO��Ũ��____________��
��4����������NaBr��Һ��������Ӧ�����ӷ���ʽ��ʾΪ____��HCl�����ʵ���_____��
��5����������ŨƷ����Һ��ʵ������Ϊ_____________��HCl�����ʵ���___________��
��6��������ˮϡ�ͣ�HClO��Ũ��__________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
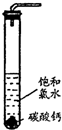 ʵ��һ������̽����
ʵ��һ������̽���� HClO+HCl�����������CaCO3����Һ�е�HCl������Ӧ��ʹ�����Ũ�ȼ�С��ƽ��������Ӧ�����ƶ�����������HClO
HClO+HCl�����������CaCO3����Һ�е�HCl������Ӧ��ʹ�����Ũ�ȼ�С��ƽ��������Ӧ�����ƶ�����������HClO HClO+HCl�����������CaCO3����Һ�е�HCl������Ӧ��ʹ�����Ũ�ȼ�С��ƽ��������Ӧ�����ƶ�����������HClO
HClO+HCl�����������CaCO3����Һ�е�HCl������Ӧ��ʹ�����Ũ�ȼ�С��ƽ��������Ӧ�����ƶ�����������HClO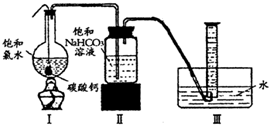
| BL |
| 22.4L/mol |
| Ag |
| 100g/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣���ˮ�д�������ƽ�⣺Cl2 + H2O ⇌ HCl+ HClO������ˮ�м����������ʣ��ش�����⣺
��1����������CaCO3(s)��������Ӧ�����ӷ���ʽ��ʾΪ__________________��ƽ����________�ƶ��������������������ͬ����HClO��Ũ��______�����������С�����䡱����ͬ����
��2����������NaCl(s)��ƽ����_________�ƶ���HClO��Ũ��___________��
��3����������NaOH(s)��ƽ����__________�ƶ���HClO��Ũ��____________��
��4����������NaBr��Һ��������Ӧ�����ӷ���ʽ��ʾΪ____��HCl�����ʵ���_____��
��5����������ŨƷ����Һ��ʵ������Ϊ_____________��HCl�����ʵ���___________��
��6��������ˮϡ�ͣ�HClO��Ũ��__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ�߶���ѧ�����п������⻯ѧ ���ͣ������
��12�֣���ˮ�д�������ƽ�⣺Cl2 + H2O ⇌ HCl+ HClO������ˮ�м����������ʣ��ش�����⣺
��1����������CaCO3(s)��������Ӧ�����ӷ���ʽ��ʾΪ__________________��ƽ����________�ƶ��������������������ͬ����HClO��Ũ��______�����������С�����䡱����ͬ����
��2����������NaCl(s)��ƽ����_________�ƶ���HClO��Ũ��___________��
��3����������NaOH(s)��ƽ����__________�ƶ���HClO��Ũ��____________��
��4����������NaBr��Һ��������Ӧ�����ӷ���ʽ��ʾΪ____��HCl�����ʵ���_____��
��5����������ŨƷ����Һ��ʵ������Ϊ_____________��HCl�����ʵ���___________��
��6��������ˮϡ�ͣ�HClO��Ũ��__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮ�д�������ƽ�⣺Cl2 + H2O ?? HCl+ HClO������ˮ�м����������ʣ��ش�����⣺
��1����������CaCO3(s)��������Ӧ�����ӷ���ʽ��ʾΪ__________________��ƽ����________�ƶ��������������������ͬ����HClO��Ũ��______�����������С�����䡱����ͬ����
��2����������NaCl(s)��ƽ����_________�ƶ���HClO��Ũ��___________��
��3����������NaOH(s)��ƽ����__________�ƶ���HClO��Ũ��____________��
��4����������NaBr��Һ��������Ӧ�����ӷ���ʽ��ʾΪ____��HCl�����ʵ���_____��
��5����������ŨƷ����Һ��ʵ������Ϊ_____________��HCl�����ʵ���___________��
��6��������ˮϡ�ͣ�HClO��Ũ��__________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com