
£®15∑÷£©£®1£©3.01°¡1022∏ˆOH¯µƒŒÔ÷ µƒ¡øŒ™ £ª’‚–©OH¯”Î molNH3µƒ÷ ¡øœýµ»£¨”Î °° g Na+∫¨”–µƒ¿Î◊” ˝œýÕ¨°£
£®2£©‘⁄±Í◊º◊¥øˆœ¬£¨2.24LNOx∆¯Ãµƒ÷ ¡øŒ™4.6g£¨‘Úxµƒ÷µŒ™ °£
£®3£©”–Àƒ÷÷’˝—ŒµƒªÏ∫œ»Ð“∫£¨∫¨”–0.2 mol°§L£≠1 Na+°¢0.25 mol°§L£≠1 Mg2+°¢0.4mol°§L£≠1
Cl£≠£¨‘ÚSO µƒ≈®∂»Œ™°° °°
µƒ≈®∂»Œ™°° °°
£®4£©°¢”√14.2g¡ÚÀ·ƒ∆≈‰÷∆≥…500mL»Ð“∫£¨∆‰ŒÔ÷ µƒ¡ø≈®∂»Œ™ mol/L°£»Ù¥”÷–»°≥ˆ50mL£¨∆‰ŒÔ÷ µƒ¡ø≈®∂»Œ™ mol/L£¨»Ð“∫÷–∫¨Na+µƒ∏ˆ ˝Œ™
»ÙΩ´’‚50mL»Ð“∫”√ÀÆœ° ÕµΩ100mL£¨ SO42-µƒŒÔ÷ µƒ¡ø≈®∂»Œ™ mol/L°£
£®1£©0.05£¨ 0.05£¨1.15 £®√øø’1∑÷£©
£®2£©2 £®2∑÷£© £®3£©0.15mol/L £®2∑÷£©
(4) 0.2 mol/L°¢ 0.02NA°¢0.2mol/L°¢0.1 mol/L°££®√øø’2∑÷£©
°æΩ‚Œˆ°ø£®1£©3.01°¡1022∏ˆOH¯∑÷◊”µƒŒÔ÷ µƒ¡ø «3.01°¡1022°¬6.02°¡1023/mol£Ω0.05mol£¨∞±∆¯µƒƒ¶∂˚÷ ¡ø”ÎOH¯µƒƒ¶∂˚÷ ¡øœýÕ¨£¨À˘“‘∞±∆¯µƒŒÔ÷ µƒ¡ø «0.05mol°£ƒ∆¿Î◊”µƒŒÔ÷ µƒ¡ø «0.05mol£¨÷ ¡ø «0.05mol°¡23g/mol£Ω1.15g°£
£®2£©±Í◊º◊¥øˆœ¬£¨2.24LNOx∆¯ÃµƒŒÔ÷ µƒ¡ø «0.1mol£¨‘Ú∏√∆¯Ãµƒœý∂‘∑÷◊”÷ ¡ø «46£¨À˘“‘x£Ω2°£
£®3£©»Ð“∫ «µÁ÷––‘µƒ£¨À˘“‘SO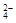 µƒ≈®∂»Œ™£®0.2
mol°§L£≠1 £´2°¡0.25 mol°§L£≠1 £≠0.4mol°§L£≠1£©°¬2£Ω0.15mol/L°£
µƒ≈®∂»Œ™£®0.2
mol°§L£≠1 £´2°¡0.25 mol°§L£≠1 £≠0.4mol°§L£≠1£©°¬2£Ω0.15mol/L°£
£®4£©14.2g¡ÚÀ·ƒ∆µƒŒÔ÷ µƒ¡ø «14.2g°¬142g/mol£Ω0.1mol£¨‘Ú∏˘æðc£Ωn/Vø…÷™£¨≈®∂» «0.1mol°¬0.5L£Ω0.2 mol/L£ª»Ð“∫ «æ˘“ªµƒ£¨“Ú¥À≈®∂»≤ª±‰£ª»Ð“∫÷–∫¨Na+µƒ∏ˆ ˝Œ™0.05L°¡0.4mol/L°¡NA£Ω0.02NA£ª‘⁄œ° Õπ˝≥Ã÷–£¨»Ð÷ «≤ª±‰µƒ£¨À˘“‘œ° Õ∫Ûµƒ≈®∂» «0.2mol/L°¬2£Ω0.1 mol/L°£


 ÃÏÃϜڅœ“ª±æ∫√æÌœµ¡–¥∞∏
ÃÏÃϜڅœ“ª±æ∫√æÌœµ¡–¥∞∏ –°—ß…˙10∑÷÷””¶”√Âœµ¡–¥∞∏
–°—ß…˙10∑÷÷””¶”√Âœµ¡–¥∞∏
| ƒÍº∂ | ∏þ÷–øŒ≥à | ƒÍº∂ | ≥ı÷–øŒ≥à |
| ∏þ“ª | ∏þ“ª√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı“ª | ≥ı“ª√‚∑—øŒ≥ÃÕ∆ºˆ£° |
| ∏þ∂˛ | ∏þ∂˛√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı∂˛ | ≥ı∂˛√‚∑—øŒ≥ÃÕ∆ºˆ£° |
| ∏þ»˝ | ∏þ»˝√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı»˝ | ≥ı»˝√‚∑—øŒ≥ÃÕ∆ºˆ£° |
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫2011-2012—߃Íπ„∂´ °π„÷ð –¡˘÷–∏þ∂˛…œ—ß∆⁄∆⁄÷–øº ‘ªØ—ߣ®Œƒ£© ‘æÌ Ã‚–Õ£∫µ•—°Ã‚
œ¬¡–Àµ∑®’˝»∑µƒ «
| A£Æ0.25 mol CO2 | B£Æ1 mol–°¬Û∫¨”–6.02°¡10 23∏ˆ¬Û¡£ |
| C£Æ1 mol —ı | D£Æ0.5 mol H2 ∫¨”– 3.01°¡10 23 ∏ˆ«‚‘≠◊” |
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫2016ΩÏÀƒ¥® °∏þ“ª…œ—ß∆⁄∆⁄÷–øº ‘ªØ—ß ‘æÌ£®Ω‚Œˆ∞Ê£© –գ∫ÃÓø’Â
£®1£©3.01°¡1022∏ˆOH£≠ µƒŒÔ÷ µƒ¡øŒ™________£¨÷ ¡øŒ™________£¨∫¨”–÷ ◊”µƒŒÔ÷ µƒ¡øŒ™________£¨∫¨”–µÁ◊”µƒŒÔ÷ µƒ¡øŒ™________°£
£®2£©œýÕ¨◊¥øˆœ¬£¨10mL X2∆¯Ã”Î30mL Y2∆¯ÃªØ∫œ…˙≥…20mL C∆¯Ã£¨‘ÚCµƒªØ—ß ΩŒ™_________°£
£®3£©ƒ≥µÿÀ·”Íæ≠ºÏ—È£¨≥˝∫¨«‚¿Î◊”Õ‚£¨ªπ”–»Áœ¬¿Î◊”£∫c(Na+)=1.4°¡10-3 mol°§L-1 £¨
c(Cl£≠ ) =3.5°¡10-3 mol°§L-1 £¨c(NH4+) =2.3°¡10-3 mol°§L-1 £¨ c(SO42-) =1.5°¡10-3
mol°§L-1°£‘Ú∏√»Ð“∫÷–«‚¿Î◊”µƒ≈®∂»Œ™_________°£
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫2013ΩÏπ„∂´ °π„÷ð –∏þ∂˛…œ—ß∆⁄∆⁄÷–øº ‘ªØ—ߣ®Œƒ£© ‘æÌ Ã‚–Õ£∫—°‘ÒÂ
œ¬¡–Àµ∑®’˝»∑µƒ «
A. 0.25 mol CO2 B. 1 mol–°¬Û∫¨”–6.02°¡10 23∏ˆ¬Û¡£
C. 1 mol —ı D. 0.5 mol H2 ∫¨”– 3.01°¡10 23 ∏ˆ«‚‘≠◊”
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
∞Ÿ∂»÷¬–≈ - ¡∑œ∞≤·¡–±Ì - ‘¡–±Ì
∫˛±± °ª•¡™Õ¯Œ•∑®∫Õ≤ª¡º–≈œ¢æŸ±®∆Ωî | Õ¯…œ”–∫¶–≈œ¢æŸ±®◊®«¯ | µÁ–≈’©∆≠柱®◊®«¯ | …Ê¿˙ ∑–ÈŒÞ÷˜“”–∫¶–≈œ¢æŸ±®◊®«¯ | …Ê∆Û«÷»®æŸ±®◊®«¯
Œ•∑®∫Õ≤ª¡º–≈œ¢æŸ±®µÁª∞£∫027-86699610 柱®” œ‰£∫58377363@163.com