


 ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ���Ĵ�ʡ�ɶ�����УЭ����2011��2012ѧ��߶���ѧ������������ѧ���� ���ͣ�013
|
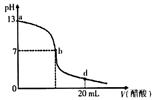
��20 mL����������Һ����μ���0.2 mol/L������Һ���ζ���������ͼ��ʾ����˵������ȷ����
| |
| [����] | |
A�� |
������������Һ�����ʵ���Ũ��Ϊ0.1 mol/L�� |
B�� |
��b�㣬c(Na+)��c(CH3COO��) |
C�� |
��d�㣬��Һ����������Ũ���ɴ�С��˳��Ϊc(CH3COO��)��c(Na+)��c(H+)��c(OH��) |
D�� |
����������Һ�������Һǡ����ȫ��Ӧ�ĵ�λ������b��d���ij�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�人�������2009�����Ԫ�µ��п��Ի�ѧ���� ���ͣ�022
�����£���20 mL����������Һ����μ���0.2 mol/L������Һ���ζ���������ͼ��ʾ��
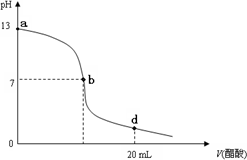
(1)������������Һ�����ʵ���Ũ��Ϊ________��
(2)��b�㣬c(Na+)________c(CH3COO��)(�����������������)��
(3)����������Һ�������Һǡ����ȫ��Ӧ�ĵ�λ�����ߵ�________(��ѡ��ı��)��
A��a��
B��b��
C��d��
D��a��b���ij��
E��b��d���ij��
(4)��d�㣬��Һ����������Ũ���ɴ�С��˳��Ϊ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
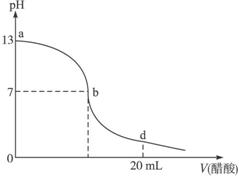
(1)������������Һ�����ʵ���Ũ��Ϊ___________��
(2)��b�㣬c(Na+)__________c(CH3COO-)(�������������=��)��
(3)����������Һ�������Һǡ����ȫ��Ӧ�ĵ�λ�����ߵ�__________(��ѡ��ı��)��
A.a�� B.b��
C.d�� D.a��b���ij��
E.b��d ���ij��
(4)��d�㣬��Һ����������Ũ���ɴ�С��˳��Ϊ_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ĵ�ʡ�ɶ�����УЭ����߶���ѧ������������ѧ�Ծ����������� ���ͣ���ѡ��
��20 mL����������Һ����μ���0. 2 mol/L������Һ���ζ���������ͼ��ʾ����˵������ȷ���� �� �� 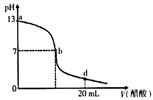
| A��������������Һ�����ʵ���Ũ��Ϊ0.1 mol/L �� |
| B����b�㣬c (Na+��=c(CH3COO-�� |
| C����d�㣬��Һ����������Ũ���ɴ�С��˳��Ϊ c (CH3COO-��>c (Na+)>c (H+��>c (OH-�� |
| D������������Һ�������Һǡ����ȫ��Ӧ�ĵ�λ������b��d���ij�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com