
£ØŃ”×öĢā£©£Ø19·Ö£©Ķ¼±ķ·Ø”¢Ä£ŠĶ·ØŹĒ³£ÓƵÄæĘѧъ¾æ·½·Ø”£
I£®ĻĀĶ¼ŹĒŃŠ¾æ²æ·ÖŌŖĖŲµÄĒā»ÆĪļµÄ·Šµć±ä»Æ¹ęĀɵÄĶ¼Ļń”£²»Ķ¬Ķ¬Ń§¶ŌijÖ÷×åŌŖĖŲĒā»ÆĪļµÄ·ŠµćµÄ±ä»ÆĒ÷ŹĘ»³öĮĖĮ½ĢõÕŪĻß”Ŗ”ŖÕŪĻßaŗĶÕŪĻßb£ØĘäÖŠAµć¶ŌÓ¦µÄ·ŠµćŹĒ100”ę£©£¬ÄćČĻĪŖÕżČ·µÄŹĒ £¬ĄķÓÉŹĒ ”£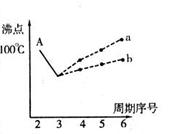
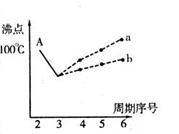
II£®ČĖĄąŌŚŹ¹ÓĆ½šŹōµÄĄśŹ·½ų³ĢÖŠ£¬¾ĄśĮĖĶ”¢Ģś”¢ĀĮÖ®ŗ󣬵ŚĖÄÖÖ½«±»¹ć·ŗÓ¦ÓĆµÄ½šŹōŌņ±»æĘѧ¼ŅŌ¤²āĪŖŹĒīŃ£ØTi£©”£īѱ»ÓžĪŖ”°Ī“Ą“ŹĄ¼ĶµÄ½šŹō”±”£ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©22TiŌŖĖŲ»łĢ¬Ō×ӵļŪµē×Ó²ćÅŲ¼Ź½ĪŖ ”£
£Ø2£©ŌŚTiµÄ»ÆŗĻĪļÖŠ£¬æÉŅŌ³ŹĻÖ+2”¢+3”¢+4ČżÖÖ»ÆŗĻ¼Ū£¬ĘäÖŠŅŌ+4¼ŪµÄTi×īĪŖĪČ¶Ø”£Ę«īŃĖį±µµÄČČĪČ¶ØŠŌŗĆ£¬¼Ūµē³£Źżøߣ¬ŌŚŠ”ŠĶ±äŃ¹Ę÷”¢»°Ķ²ŗĶĄ©ŅōĘ÷ÖŠ¶¼ÓŠÓ¦ÓĆ”£Ę«īŃĖį±µ¾§ĢåÖŠ¾§°ūµÄ½į¹¹Ź¾ŅāĶ¼ČēÓŅĶ¼£¬ŌņĖüµÄ»ÆѧŹ½ŹĒ ”£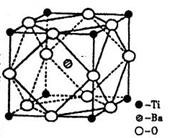
III£®ÉĻŹĄ¼Ķ60Äź»Æ£¬µŚŅ»øöĻ”ÓŠĘųĢå»ÆŗĻĪļXe[PtF6]±»ŗĻ³É³öĄ“ŗ󣬓ņĘĘĮĖ”°¾ų¶ŌĒéŠŌ”±µÄ¹ŪÄī”£ŌŚĖęŗóµÄ¼øğČ£¬æĘѧ¼ŅÓÖĻą¼Ģŗó³ÉĮĖėƵķś»ÆĪļ”¢Ńõ»ÆĪļµČ”£
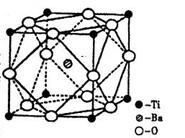
£Ø1£©½šŹōPtÄŚ²æŌ×ӵĶѻż·½Ź½ÓėĶ¼°øɱłÖŠµÄCO2ĻąĶ¬£¬ÓŅĶ¼Õż·½ĢåŹĒPt¾§°ūµÄŹ¾ŅāĶ¼£¬ŹŌĖµ³öPtŌ×ÓŌŚ¾§°ūÖŠµÄĪ»ÖĆ ”£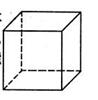
£Ø2£©Ļ”ÓŠĘųĢå£Øė±³żĶā£©ÖŠ£¬Ö»ÓŠ½ĻÖŲµÄėÆÄÜŗĻ³É³ö¶ąÖÖ»ÆŗĻĪļ£¬
ĘäæÉÄÜŌŅņŹĒ £ØĢī×ÖÄø“śŗÅ£©
| A£®ėƵÄŗ¬Įæ±Č½Ļ·įø» | B£®ėƵÄĻą¶ŌŌ×ÓÖŹĮæ“ó |
| C£®ėÆŌ×Ó°ė¾¶“󣬵ēĄėÄÜŠ” | D£®ėÆŌ×Ó°ė¾¶Š”£¬µēøŗŠŌ“ó |
 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
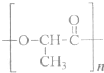


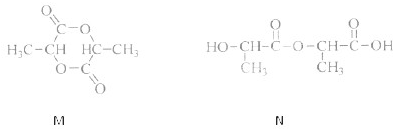
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
1.”¶ÓŠ»ś»Æѧ»ł“””·
ŅŃÖŖ£ŗĮ½øöōĒ»łĶ¬Ź±Į¬ŌŚĶ¬Ņ»Ģ¼Ō×ÓÉĻµÄ½į¹¹ŹĒ²»ĪČ¶ØµÄ£¬Ėü½«·¢ÉśĶŃĖ®·“Ó¦£ŗ

ĻÖÓŠ·Ö×ÓŹ½ĪŖC9H8O2Br2µÄĪļÖŹM£¬ÓŠ»śĪļCµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ60£¬ŌŚŅ»¶ØĢõ¼žĻĀæÉ·¢ÉśĻĀŹöŅ»ĻµĮŠ·“Ó¦£ŗ

Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)G”śHµÄ·“Ó¦ĄąŠĶŹĒ_______________”£
(2)MµÄ½į¹¹¼ņŹ½ĪŖ_______________£»CµÄ½į¹¹¼ņŹ½ĪŖ_______________”£
(3)Š“³öĻĀĮŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
¢ŁA”śBµÄ»Æѧ·½³ĢŹ½£ŗ________________________________________________________£»
¢ŚH”śIµÄ»Æѧ·½³ĢŹ½£ŗ_________________________________________________________”£
2.”¶ĪļÖŹ½į¹¹ÓėŠŌÖŹ”·¢ń.Ķ¼±ķ·Ø”¢Ķ¼Ļń·ØŹĒ³£ÓƵÄæĘѧъ¾æ·½·Ø”£


Ķ¼A Ķ¼B
(1)¶ĢÖÜĘŚÄ³Ö÷×åŌŖĖŲMµÄµēĄėÄÜĒéæöČēĶ¼AĖłŹ¾”£ŌņMŌŖĖŲĪ»ÓŚÖÜĘŚ±ķµÄµŚ_____×唣
(2)Ķ¼BŹĒŃŠ¾æ²æ·ÖŌŖĖŲµÄĒā»ÆĪļµÄ·Šµć±ä»Æ¹ęĀɵÄĶ¼Ļń£¬ÕŪĻßcæÉŅŌ±ķ“ļ³öµŚ_____×åŌŖĖŲĒā»ÆĪļµÄ·ŠµćµÄ±ä»Æ¹ęĀÉ”£²»Ķ¬Ķ¬Ń§¶ŌijÖ÷×åŌŖĖŲĒā»ÆĪļµÄ·ŠµćµÄ±ä»ÆĒ÷ŹĘ»³öĮĖĮ½ĢõÕŪĻß”Ŗ”ŖÕŪĻßaŗĶÕŪĻßb£¬ÄćČĻĪŖÕżČ·µÄŹĒ_____(Ģī”°a”±»ņ”°b”±)”£
¢ņ.ČĖĄąŌŚŹ¹ÓĆ½šŹōµÄĄśŹ·½ų³ĢÖŠ£¬¾ĄśĮĖĶ”¢Ģś”¢ĀĮÖ®ŗ󣬵ŚĖÄÖÖ½«±»¹ć·ŗÓ¦ÓĆµÄ½šŹō±»æĘѧ¼ŅŌ¤²āĪŖŹĒīŃ(22Ti)£¬Ėü±»ÓžĪŖ”°Ī“Ą“ŹĄ¼ĶµÄ½šŹō”±”£ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
(3)TiŌŖĖŲµÄ»łĢ¬Ō×ӵļŪµē×Ó²ćÅŲ¼Ź½ĪŖ____________”£
(4)ŌŚTiµÄ»ÆŗĻĪļÖŠ£¬æÉŅŌ³ŹĻÖ+2”¢+3”¢+4ČżÖÖ»ÆŗĻ¼Ū£¬ĘäÖŠŅŌ+4¼ŪµÄTi×īĪŖĪČ¶Ø£»Ę«īŃĖį±µµÄČČĪČ¶ØŠŌŗĆ£¬½éµē³£Źżøߣ¬ŌŚŠ”ŠĶ±äŃ¹Ę÷”¢»°Ķ²ŗĶĄ©ŅōĘ÷ÖŠ¶¼ÓŠÓ¦ÓĆ”£Ę«īŃĖį±µ¾§ĢåÖŠ¾§°ūµÄ½į¹¹Ź¾ŅāĶ¼ČēĻĀĶ¼£¬ĖüµÄ»ÆѧŹ½ŹĒ__________£»¾§ĢåÄŚÓėĆæøö”°Ti”±½ōĮŚµÄŃõŌ×ÓŹżĪŖ_________øö”£

(5)ŅŃÖŖTi3+æÉŠĪ³ÉÅäĪ»ŹżĪŖ6µÄÅäŗĻĪļ”£ĻÖÓŠŗ¬īѵÄĮ½ÖÖŃÕÉ«µÄ¾§Ģ壬Ņ»ÖÖĪŖ×ĻÉ«£¬ĮķŅ»ÖÖĪŖĀĢÉ«£¬µ«Ļą¹ŲŹµŃéÖ¤Ć÷£¬Į½ÖÖ¾§ĢåµÄ×é³É½ŌĪŖTiCl3”¤6H2O”£ĪŖ²ā¶ØÕāĮ½ÖÖ¾§ĢåµÄ»ÆѧŹ½£¬Éč¼ĘĮĖČēĻĀŹµŃé£ŗ
a.·Ö±šČ”µČÖŹĮæµÄĮ½ÖÖÅäŗĻĪļ¾§ĢåµÄѳʷÅä³É“ż²āČÜŅŗ£»b.·Ö±šĶł“ż²āČÜŅŗÖŠµĪČėAgNO3ČÜŅŗ£¬¾ł²śÉś°×É«³Įµķ£»c.³ĮµķĶźČ«ŗó·Ö±š¹żĀĖµĆĮ½·Ż³Įµķ£¬¾Ļ“µÓøÉŌļŗó³ĘĮ棬·¢ĻÖŌĀĢÉ«¾§ĢåµÄĖ®ČÜŅŗÓėAgNO3ČÜŅŗ·“Ó¦µĆµ½µÄ°×É«³ĮµķÖŹĮæĪŖ×ĻÉ«¾§ĢåµÄĖ®ČÜŅŗ·“Ó¦µĆµ½³ĮµķÖŹĮæµÄ![]() ”£ŌņĀĢÉ«¾§ĢåÅäŗĻĪļµÄ»ÆѧŹ½ĪŖ_______________”£
”£ŌņĀĢÉ«¾§ĢåÅäŗĻĪļµÄ»ÆѧŹ½ĪŖ_______________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
(A)”¾ĪļÖŹ½į¹¹ÓėŠŌÖŹ”æ
ĻĀ±ķŹĒŌŖĖŲÖÜĘŚ±ķµÄŅ»²æ·Ö”£±ķÖŠĖłĮŠµÄ×ÖÄø·Ö±š“ś±ķijŅ»ÖÖ»ÆѧŌŖĖŲ”£

(1)T3+µÄŗĖĶāµē×ÓÅŲ¼Ź½ŹĒ____________”£
(2)Q”¢R”¢MµÄµŚŅ»µēĄėÄÜÓɓ󵽊”µÄĖ³ŠņŹĒ___________________(ÓĆŌŖĖŲ·ūŗűķŹ¾)”£
(3)ĻĀĮŠÓŠ¹ŲÉĻŹöŌŖĖŲµÄĖµ·ØÖŠ£¬ÕżČ·µÄŹĒ______________________(ĢīŠņŗÅ)”£
¢ŁGµ„ÖŹµÄČŪµćøßÓŚJµ„ÖŹ£¬ŹĒŅņĪŖGµ„ÖŹµÄ½šŹō¼ü½ĻĒæ
¢ŚJ±ČX»īĘĆ£¬ĖłŅŌJæÉŅŌŌŚČÜŅŗÖŠÖĆ»»³öX
¢Ū½«J
¢ÜRE3·ŠµćøßÓŚQE4£¬Ö÷ŅŖŹĒŅņĪŖĒ°ÕßĻą¶Ō·Ö×ÓÖŹĮæ½Ļ“ó
¢ŻŅ»øöQ2E4·Ö×ÓÖŠŗ¬ÓŠĪåøö¦Ņ¼üŗĶŅ»øö¦Š¼ü
(4)¼ÓÄĆ“óĢģĪÄĢØŌŚĢ«æÕ·¢ĻÖĮĖEQ9R£¬ŅŃÖŖ·Ö×ÓÖŠĖłÓŠŌ×Ó¾łŠĪ³É8µē×Ó»ņ2µē×ÓĪČ¶Ø½į¹¹£¬ŹĒÖ±ĻߊĪ·Ö×Ó£¬²»“ęŌŚÅäĪ»¼ü”£Š“³öĘä½į¹¹Ź½£ŗ_________________”£
(5)GÓėRµ„ÖŹÖ±½Ó»ÆŗĻÉś³ÉŅ»ÖÖĄė×Ó»ÆŗĻĪļG3R”£øĆ¾§Ģå¾ßÓŠĄąĖĘŹÆÄ«µÄ²ćד½į¹¹”£Ćæ²ćÖŠ£¬GŌ×Ó¹¹³ÉĘ½ĆęĮł±ßŠĪ£¬ĆæøöĮł±ßŠĪµÄÖŠŠÄÓŠŅ»øöRŌ×Ó”£²ćÓė²ćÖ®¼ä»¹¼ŠŌÓŅ»¶ØŹżĮæµÄŌ×Ó”£ĒėĪŹÕāŠ©¼ŠŌÓµÄŌ×ÓÓ¦øĆŹĒ____________(ĢīG»ņRµÄŌŖĖŲ·ūŗÅ)”£

(B)”¾ŹµŃé»Æѧ”æ
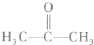
Ä³×ŹĮĻĻŌŹ¾£¬ÄÜŹ¹Ė«ŃõĖ®·Ö½āµÄ“߻ƼĮÓŠŗܶąÖÖ£¬ÉśĪļ“߻ƼĮ(ČēÖķøĪ)”¢Ąė×ÓŠĶ“߻ƼĮ(ČēFeCl3)ŗĶ¹ĢĢå“߻ƼĮ(ČēMnO2)µČ¶¼ŹĒ½ĻŗĆµÄ“ß»Æ¼Į”£Ä³ŹµŃ銔×éĶعż²ā¶ØĖ«ŃõĖ®·Ö½ā²śÉśµÄO2µÄŃ¹Ē棬Ģ½¾æ·Ö½ā¹żŃõ»ÆĒāµÄ×ī¼Ń“߻ƼĮŅŌ¼°Ģ½¾æ×ī¼Ń“߻ƼĮŗĻŹŹµÄ“ß»ÆĢõ¼ž”£
(Ņ»)Ģ½¾æŅ»£ŗ
ŹµŃé²½Öč
(1)Ķł×¶ŠĪĘæÖŠ¼ÓČė50 mL 1.5£„µÄĖ«ŃõĖ®
(2)·Ö±šĶł×¶ŠĪĘæÖŠ¼Ó
(3)²É¼ÆŗĶ¼ĒĀ¼Źż¾Ż”£
(4)ÕūĄķŹż¾ŻµĆ³öĻĀ±ķ
²»Ķ¬“߻ƼĮ”°Ń¹Ēæ¶ŌŹ±¼äŠ±ĀŹ”±µÄ±Č½Ļ
“߻ƼĮ | ÖķøĪ | ĀķĮåŹķ | ĀČ»ÆĶ | ĀČ»ÆĢś | Ńõ»ÆĶ | ¶žŃõ»ÆĆĢ |
Ń¹Ēæ¶ŌŹ±¼äµÄŠ±ĀŹ | 0.191 87 | 0.002 42 | 0.007 93 | 0.030 5 | 0.015 47 | 1.833 6 |
¢ŁøĆ”°Ģ½¾æŅ»”±ŹµŃéµÄĆū³ĘŹĒ_____________________________________________________”£
¢ŚøĆŹµŃéĖłµĆ³öµÄ½įĀŪŹĒ_______________________________________________________”£
(¶ž)Ģ½¾æ¶ž£ŗ¶žŃõ»ÆĆĢ“߻ƵÄ×ī¼Ń“ß»ÆĢõ¼ž
øĆŹµŃ銔×éµÄĶ¬Ń§ŌŚ½ųŠŠĢ½¾æ¶žµÄŹµŃ鏱£¬µĆµ½ĮĖŅ»ĻµĮŠµÄĶ¼±ķŗĶŹż¾Ż”£²Īæ“ĻĀĶ¼ŗĶ±ķøń·Ö±š»Ų“šĻą¹ŲĪŹĢā”£

3%µÄĖ«ŃõĖ®Óė²»Ķ¬ÓĆĮ涞Ńõ»ÆĆĢµÄŃ¹Į¦”ŖŹ±¼äĶ¼
±ķ£ŗ²»Ķ¬ÅØ¶ČµÄĖ«ŃõĖ®ŌŚ²»Ķ¬ÓĆĮæµÄ¶žŃõ»ÆĆĢ×÷ÓĆĻĀŹÕ¼ÆĻąĶ¬×“æöĻĀĶ¬Ģå»żO2ĖłŠčŹ±¼ä
MnO2 Ź±¼ä H2O2 | |||
1.5£„ | 223 s | 67 s | 56 s |
3.0£„ | 308 s | 109 s | 98 s |
4.5£„ | 395 s | 149 s | 116 s |
·ÖĪöĶ¼”¢±ķÖŠŹż¾ŻĪŅĆĒæÉŅŌµĆ³ö£ŗ
¢ŪĶ¬ÅØ¶ČµÄĖ«ŃõĖ®µÄ·Ö½āĖŁĀŹĖę×ŶžŃõ»ÆĆĢÓĆĮæµÄŌö¼Ó¶ų_________________£¬Ņņ¶ų·“Ó¦Ź±¼ä_______________”£
¢ÜČē¹ū“ÓŹµŃé½į¹ūŗĶ½ŚŹ”Ņ©Ę·µÄ½Ē¶Č×ŪŗĻ·ÖĪö£¬ÄćČĻĪŖµ±ĪŅĆĒŃ”ÓĆ3.0%µÄĖ«ŃõĖ®£¬¼ÓČė___________ gµÄ¶žŃõ»ÆĆĢÄÜŹ¹ŹµŃ銧¹ū×ī¼Ń”£ÄćÅŠ¶ĻµÄĄķÓÉŹĒ______________________”£
¢ŻøĆŠ”×éµÄijĶ¬Ń§Ķعż·ÖĪöŹż¾ŻµĆ³öĮĖµ±“߻ƼĮÓĆĮæĻąĶ¬Ź±Ė«ŃõĖ®µÄÅضČŌ½Š”·“Ó¦ĖŁĀŹŌ½æģµÄ½įĀŪ£¬ÄćČĻĪŖŹĒ·ńÕżČ·____________£¬ÄćµÄĄķÓÉŹĒ________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
£ØŃ”×öĢā£©Ķ¼±ķ·Ø”¢Ä£ŠĶ·ØŹĒ³£ÓƵÄæĘѧъ¾æ·½·Ø”£
I£®ĻĀĶ¼ŹĒŃŠ¾æ²æ·ÖŌŖĖŲµÄĒā»ÆĪļµÄ·Šµć±ä»Æ¹ęĀɵÄĶ¼Ļń”£²»Ķ¬Ķ¬Ń§¶ŌijÖ÷×åŌŖĖŲĒā»ÆĪļµÄ·ŠµćµÄ±ä»ÆĒ÷ŹĘ»³öĮĖĮ½ĢõÕŪĻß”Ŗ”ŖÕŪĻßaŗĶÕŪĻßb£ØĘäÖŠAµć¶ŌÓ¦µÄ·ŠµćŹĒ100”ę£©£¬ÄćČĻĪŖÕżČ·µÄŹĒ £¬ĄķÓÉŹĒ ”£
II£®ČĖĄąŌŚŹ¹ÓĆ½šŹōµÄĄśŹ·½ų³ĢÖŠ£¬¾ĄśĮĖĶ”¢Ģś”¢ĀĮÖ®ŗ󣬵ŚĖÄÖÖ½«±»¹ć·ŗÓ¦ÓĆµÄ½šŹōŌņ±»æĘѧ¼ŅŌ¤²āĪŖŹĒīŃ£ØTi£©”£īѱ»ÓžĪŖ”°Ī“Ą“ŹĄ¼ĶµÄ½šŹō”±”£ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©22TiŌŖĖŲ»łĢ¬Ō×ӵļŪµē×Ó²ćÅŲ¼Ź½ĪŖ ”£
£Ø2£©ŌŚTiµÄ»ÆŗĻĪļÖŠ£¬æÉŅŌ³ŹĻÖ+2”¢+3”¢+4ČżÖÖ»ÆŗĻ¼Ū£¬ĘäÖŠŅŌ+4¼ŪµÄTi×īĪŖĪČ¶Ø”£Ę«īŃĖį±µµÄČČĪČ¶ØŠŌŗĆ£¬¼Ūµē³£Źżøߣ¬ŌŚŠ”ŠĶ±äŃ¹Ę÷”¢»°Ķ²ŗĶĄ©ŅōĘ÷ÖŠ¶¼ÓŠÓ¦ÓĆ”£Ę«īŃĖį±µ¾§ĢåÖŠ¾§°ūµÄ½į¹¹Ź¾ŅāĶ¼ČēÓŅĶ¼£¬ŌņĖüµÄ»ÆѧŹ½ŹĒ ”£
III£®ÉĻŹĄ¼Ķ60Äź»Æ£¬µŚŅ»øöĻ”ÓŠĘųĢå»ÆŗĻĪļXe[PtF6]±»ŗĻ³É³öĄ“ŗ󣬓ņĘĘĮĖ”°¾ų¶ŌĒéŠŌ”±µÄ¹ŪÄī”£ŌŚĖęŗóµÄ¼øğČ£¬æĘѧ¼ŅÓÖĻą¼Ģŗó³ÉĮĖėƵķś»ÆĪļ”¢Ńõ»ÆĪļµČ”£
£Ø1£©½šŹōPtÄŚ²æŌ×ӵĶѻż·½Ź½ÓėĶ¼°øɱłÖŠµÄCO2ĻąĶ¬£¬ÓŅĶ¼Õż·½ĢåŹĒPt¾§°ūµÄŹ¾ŅāĶ¼£¬ŹŌĖµ³öPtŌ×ÓŌŚ¾§°ūÖŠµÄĪ»ÖĆ ”£
£Ø2£©Ļ”ÓŠĘųĢå£Øė±³żĶā£©ÖŠ£¬Ö»ÓŠ½ĻÖŲµÄėÆÄÜŗĻ³É³ö¶ąÖÖ»ÆŗĻĪļ£¬
ĘäæÉÄÜŌŅņŹĒ £ØĢī×ÖÄø“śŗÅ£©
A£®ėƵÄŗ¬Įæ±Č½Ļ·įø» B£®ėƵÄĻą¶ŌŌ×ÓÖŹĮæ“ó
C£®ėÆŌ×Ó°ė¾¶“󣬵ēĄėÄÜŠ” D£®ėÆŌ×Ó°ė¾¶Š”£¬µēøŗŠŌ“ó
£Ø3£©ŅŃÖŖXeO3·Ö×ÓÖŠėÆŌ×ÓÉĻÓŠ1¶Ō»”¶Ōµē×Ó£¬ŌņXeO3ĪŖ ·Ö×Ó£ØĢī”°¼«ŠŌ”±»ņ”°·Ē¼«ŠŌ”±£©”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com