
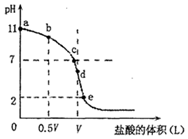
 c��NH4+��+c��H+��=c��NH3•H2O��+c��OH-��
c��NH4+��+c��H+��=c��NH3•H2O��+c��OH-������ ��1���ζ�ǰ��ˮ��c��H+��=10-11mol/L������ˮ�����ӻ���c��OH-��=$\frac{1{0}^{-14}}{1{0}^{-11}}$mol/L=10-3mol/L��һˮ�ϰ��ĵ���ƽ�ⳣ��K=$\frac{c��O{H}^{-}����c��N{{H}_{4}}^{+}��}{c��N{H}_{3}��{H}_{2}O��}$��
��2����������ˮ���룬���������ӵ��δٽ�ˮ���룬������Ũ��Խ��������ˮ����̶�Խ��
��3���ζ�ʱ����b�㵽c��Ĺ����У�����ˮ�����ӻ���������ˮ�ĵ���ƽ�⡢�Ȼ�淋�ˮ��ƽ��ֻ���¶��й���������
��4��A����ͼ���֪����b��Ӧ����Һ��NH4C1��NH3•H2O���ʵ���֮��Ϊ1��1�Ļ�����Һ�ʼ��ԣ�˵��NH3•H2O����̶ȴ���NH4C1��ˮ��̶ȣ��ɵ���غ��֪��c��C1-��+c��OH-��=c��NH4+��+c��H+���������غ�Ϊ��2c��Cl-��=c��NH4+��+c��NH3��H2O���������غ�Ϊ��2c��OH-��+c��NH3��H2O��=c��NH4+��+2c��H+����
B���ɵ���غ��֪��c��C1-��+c��OH-��=c��NH4+��+c��H+������ͼ���֪����c pH=7����c��H+��=c��OH-������c��NH4+��=c��Cl-����
C����ͼ���֪����d�����ͬ����ǡ����ȫ��Ӧ����NH4Cl�������ε�ˮ����������Һ�����ԣ���c��Cl-����c��NH4+����c��H+����c��OH-����
D����NH3•H2O�϶࣬����� HCl����ʱ������NH4C1��������Һ��NH3•H2OŨ��Զ����NH4C1Ũ�ȣ����ܳ��֣�
��5����ͼ���֪����d�����ͬ����ǡ����ȫ��Ӧ����NH4C1�����㣻�����������ϡ�ͣ�����H+�����ʵ���������м��㣮
��� �⣺��1���ζ�ǰ��ˮ��c��H+��=10-11mol/L������ˮ�����ӻ���c��OH-��=$\frac{1{0}^{-14}}{1{0}^{-11}}$mol/L=10-3mol/L��һˮ�ϰ��ĵ���ƽ�ⳣ��K=$\frac{c��O{H}^{-}����c��N{{H}_{4}}^{+}��}{c��N{H}_{3}��{H}_{2}O��}$=$\frac{1{0}^{-3}��1{0}^{-3}}{0.1-1{0}^{-3}}$=10-5��
�ʴ�Ϊ��10-5��
��2����b��ʱ������Ϊ��ˮ���Ȼ�泥���ˮ�ĵ���̶ȴ����Ȼ�淋�ˮ��̶ȣ���ҺΪ���ԣ���ˮ�ĵ���ƽ�����������ã�
��c��ʱ������Ϊ��ˮ���Ȼ�泥���ˮ�ĵ���̶ȵ������Ȼ�淋�ˮ��̶ȣ���ҺΪ���ԣ���ˮ�ĵ���ƽ����Ӱ�죬
��d��ʱ������Ϊ�Ȼ�泥��Ȼ�立���ˮ�⣬��ҺΪ���ԣ���ˮ�ĵ���ƽ��ٽ���
����b��c��d����ʱ����Һ�У�ˮ�����c��OH-����С˳����d��c��b��
�ʴ�Ϊ��d��c��b��
��3��A���¶Ȳ���ˮ�����ӻ��������䣬����c��H+��•c��OH-�����䣬��A��ȷ��
B����b�㵽c��Ĺ����У�c��H+����������c��OH-�����ϼ�С������ $\frac{{c��{H^+}��}}{{c��O{H^-}��}}$ ����B����
C.$\frac{c��N{{H}_{4}}^{+}����c��O{H}^{-}��}{c��N{H}_{3}��{H}_{2}O��}$=Kb���¶Ȳ��䣬����ƽ�ⳣ�����䣬��C��ȷ��
D���¶Ȳ��䣬����ƽ�ⳣ�������ӻ��������䣬$\frac{c��N{H}_{3}��{H}_{2}O����c��{H}^{+}��}{c��N{{H}_{4}}^{+}��}$=$\frac{c��N{H}_{3}��{H}_{2}O����c��{H}^{+}����c��O{H}^{-}��}{c��N{{H}_{4}}^{+}����c��O{H}^{-}��}$=$\frac{{K}_{W}}{{K}_{b}}$���䣬��D��ȷ��
��ѡBCD��
��4��A����ͼ���֪����b��Ӧ����Һ��NH4C1��NH3•H2O���ʵ���֮��Ϊ1��1�Ļ�����Һ�ʼ��ԣ�˵��NH3•H2O����̶ȴ���NH4C1��ˮ��̶ȣ��ɵ���غ��֪��c��C1-��+c��OH-��=c��NH4+��+c��H+���������غ�Ϊ��2c��C1-��=c��NH4+��+c��NH3��H2O���������غ�Ϊ��2c��OH-��+c��NH3��H2O��=c��NH4+��+2c��H+������A����
B����ͼ���֪����c pH=7����c��H+��=c��OH-�����ɵ���غ��֪��c��C1-��+c��OH-��=c��NH4+��+c��H+������c��NH4+��=c��C1-����c��OH-��=c��H+������B����
C����ͼ���֪����d �����ͬ����ǡ����ȫ��Ӧ����NH4C1��NH4C1ˮ����Һ�����ԣ���c��C1-����c��NH4+����c��H+����c��OH-������C����
D����NH3•H2O�϶࣬����� HCl����ʱ������NH4C1��������Һ��NH3•H2OŨ��Զ����NH4C1Ũ�ȣ����ܳ���c��NH3•H2O����c��NH4+����c��OH-����c��Cl-����c��H+������D��ȷ��
�ʴ�Ϊ��D��
��5����ͼ���֪����d�����ͬ����ǡ����ȫ��Ӧ����NH4C1����NH3•H2O��HCl��c��HCl��=$\frac{0.1mol/L��VL}{VL}$=0.1mol/L���ζ����������������pH=1��
����������ΪXL�������������Ϊ0.1mol/L��XL-0.1Vmol/L��VL����c��H+��=$\frac{0.1mol/L����X-V��L}{��X+V��L}$=10-2mol/L����ã�X=$\frac{11V}{9}$��
�ʴ�Ϊ��1��$\frac{11V}{9}$��
���� ������HCl��Һ�ζ�NH3•H2O����Ϊ���壬���������ˮ�⡢��Һ����Ũ�ȵĴ�С�Ƚ��Լ�����ȣ���Ŀ�ѶȽϴ�ע������Ũ�ȴ�С�Ƚ��е���غ㡢���Ӻ��ʽ�������غ������ϵʽ�����ã�ע�⣨3����CD��ʽ�任��Ϊ�״��㣮


 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д� ������ϵ�д�
������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������Ԫ������Ԫ�� | |
| B�� | ���������뻹ԭ��������ʵ���֮��Ϊl��1 | |
| C�� | ����l mol��ԭ����ת��5 mol���� | |
| D�� | ����1 L��CN-1.04 mg•L-1�ķ�ˮ������������2��l0-5mol C1O2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��2HI��g��?H2��g��+I2��g��ƽ����ϵ����ѹǿʹ��ɫ���� | |
| B�� | ��ӦCO��g��+NO2��g��?CO2��g��+NO��g����H��0�����¶�ʹƽ�����淽���ƶ� | |
| C�� | �ϳɰ���Ӧ��N2��g��+3H2��g��?2NH3��g����H��0��Ϊʹ���IJ�����ߣ�������Ӧ��ȡ���¸�ѹ�Ĵ�ʩ | |
| D�� | ����ˮ�д�������ƽ�⣺Br2��g��+H2O��l��?HBr��aq��+HBrO��aq����������NaOH��Һ����ɫ��dz |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ϊ��ֹ�����⣬�������Ϳһ��������� | |
| B�� | Ϊ��ֹ���֣�����۳�������վ�ȳ���Ҫ�Ͻ��̻� | |
| C�� | Ϊ�ӿ�KClO3�ķֽ����ʣ�����MnO2 | |
| D�� | Ϊ���H2O2�ķֽ��ʣ�����Һ�еμ�FeCl3��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
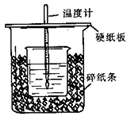 ��50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �¶�/�� | 1000 | 1150 | 1300 |
| ƽ�ⳣ�� | 64.0 | 50.7 | 42.9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
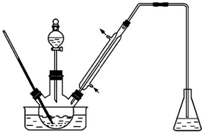 ʵ�����Ա���ȩΪԭ���Ʊ����屽��ȩ��ʵ��װ����ͼ��������ʵķе����������
ʵ�����Ա���ȩΪԭ���Ʊ����屽��ȩ��ʵ��װ����ͼ��������ʵķе����������| ���� | �е�/�� | ���� | �е�/�� |
| �� | 58.8 | 1��2-�������� | 83.5 |
| ����ȩ | 179 | ���屽��ȩ | 229 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com